Fig. 4. Structures of O2-sensitive metalloenzymes.
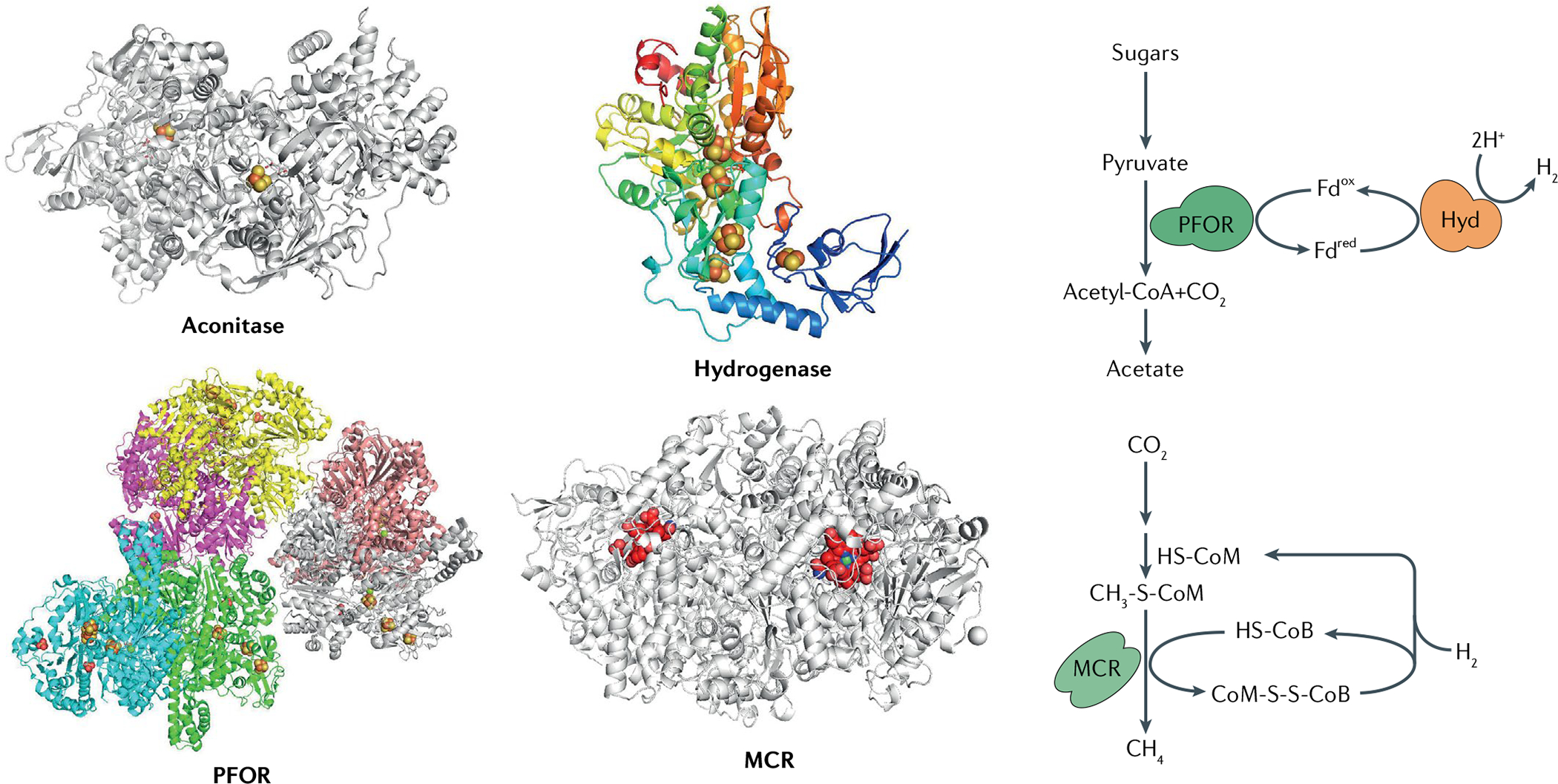
Molecular oxygen (O2) directly inactivates diverse metalloenzymes featuring low-potential metal centres at or near the protein surface. The structures of representative O2-sensitive metalloenzymes are shown (left), alongside their pivotal positions in anaerobic metabolism (right): aconitase B from Escherichia coli (Protein Data Bank (PDB) ID 1L5J); pyruvate:ferredoxin oxidoreductase (PFOR) from Moorella thermoacetica (PDB ID 6CIN); iron-only hydrogenase (Hyd) from Clostridium pasteurianum (PDB ID 1FEH); and methyl-coenzyme M reductase (MCR) from Methanothermobacter marburgensis Marburg (PDB ID 1MRO). Iron–sulfur clusters are represented by yellow and red balls, nickel-containing F430 of MCR is shown in red with nickel in green. CoM-SH, coenzyme M; CoB-SH, coenzyme B; Fdox, oxidized ferredoxin; Fdred, reduced ferredoxin.
