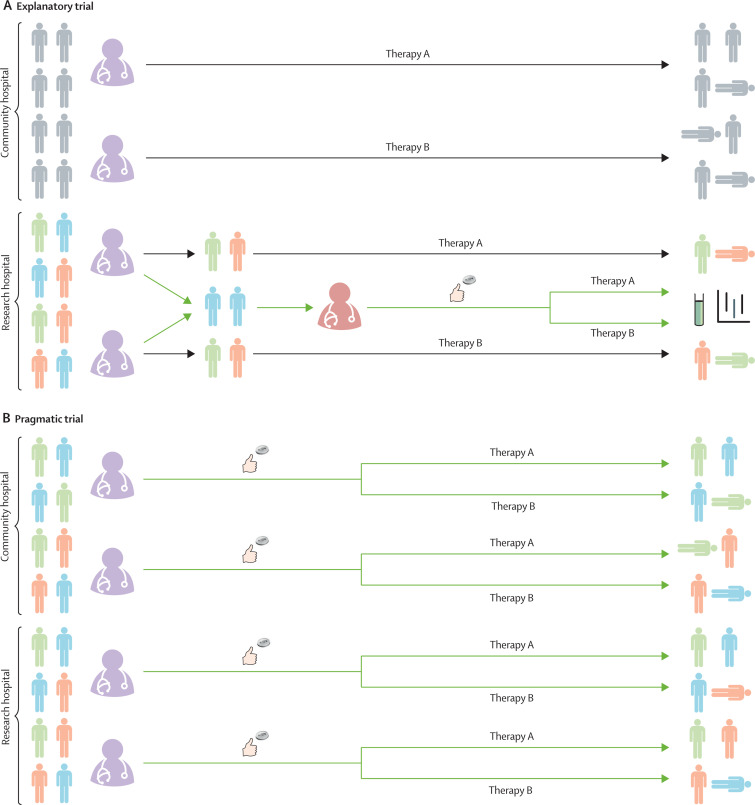
Figure 1.
Enrolment and intervention delivery in explanatory and pragmatic trials
This figure serves to highlight the differences between explanatory and pragmatic trials (green arrows) and how they impact routine clinical care (black arrows). (A) Explanatory trials. Many hospitals do not have research teams capable of conducting explanatory clinical trials (community hospital, top). When there is uncertainty regarding the relative effectiveness of two therapies, arbitrary variation develops as part of usual care in the community hospital setting. Some treating clinicians (purple) choose to use one therapy (therapy A) as part of usual care and others choose to use a different therapy (therapy B). Because this practice, which applies to all patients (grey), is not incorporated into a clinical trial, it does not generate generalisable knowledge. Conversely, in hospitals capable of conducting explanatory trials (research hospital, bottom), members of a dedicated research team (red) perform screening, enrolment, randomisation, intervention delivery, and outcome assessment among a carefully selected, homogeneous group of patients (blue) while excluding most of the patients receiving those therapies as part of usual care (green and orange). Explanatory trials are typically underpowered to detect differences in patient-centred outcomes such as mortality, and therefore focus on biomarkers or other surrogate outcomes. (B) Pragmatic trials. In contrast to the explanatory trial, pragmatic trials embed screening, enrolment, intervention delivery, and outcome assessment into routine clinical care. Using these efficient methods, pragmatic trials can enrol enough patients to detect differences in patient-centred outcomes (eg, mortality), and by enrolling all patients receiving the therapy as part of clinical care (blue, green, and orange patients at community and research hospitals), pragmatic trials provide generalisable results. While some pragmatic trials such as the RECOVERY trial also leverage a platform design (to cycle in and out proposed interventions using a shared trial infrastructure) or Bayesian sequential analysis (to ensure that enrolment is continued until trial evidence is definitive for effectiveness or futility), these aspects of trial design can be combined with both pragmatic and explanatory trials. Furthermore, the separate research teams employed in explanatory trials might be the ideal method of evaluating trials of novel therapies for which the safety profile is unknown.