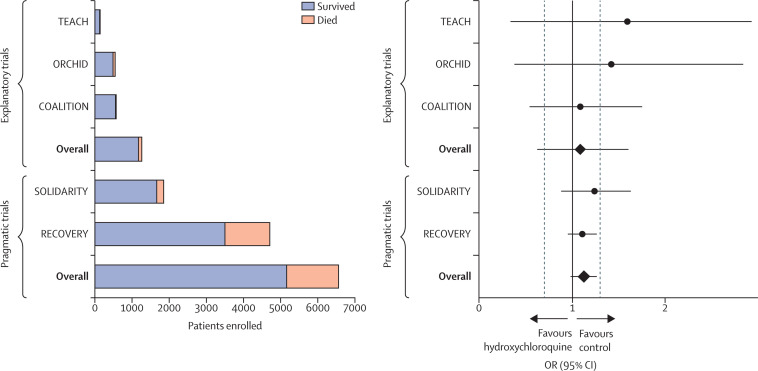
Figure 2.
Explanatory and pragmatic trials of hydroxychloroquine in COVID-19
Five trials (three explanatory and two pragmatic) that randomly assigned at least 100 patients admitted to hospital with COVID-19 to hydroxychloroquine or a control (placebo or usual care) are shown. Total enrolment by trial and treatment group are shown on the left; survival outcomes are included in the stacked bars (orange, patients who died; blue, patients who survived). The ORs and 95% CIs for mortality for each of the five trials are shown on the right. The black vertical line represents an OR of 1·0 (no effect) and the two adjacent grey dashed lines denote ORs of 0·7 (benefit from hydroxychloroquine) and 1·3 (harm from hydroxychloroquine). Each of the pragmatic trials (RECOVERY and SOLIDARITY) enrolled more patients than the three explanatory trials (TEACH, COALITION, and ORCHID) combined. COALITION=Safety and Efficacy of Hydroxychloroquine Associated With Azithromycin in SARS-CoV-2 Virus.80 OR=odds ratio. ORCHID=Outcomes Related to COVID-19 Treated With Hydroxychloroquine Among Inpatients With Symptomatic Disease.72 RECOVERY=Randomised Evaluation of COVID-19 Therapy.8, 31, 32 SOLIDARITY=Trial of Treatments for COVID-19 in Hospitalized Adults.81 TEACH=Treating COVID-19 With Hydroxychloroquine.82