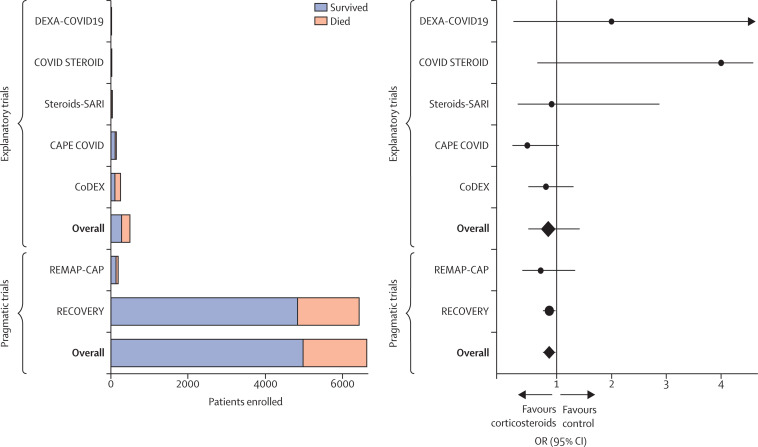
Figure 3.
Explanatory and pragmatic trials of corticosteroids in COVID-19
Seven trials (five explanatory and two pragmatic) that randomly assigned patients admitted to hospital with COVID-19 to corticosteroids or control (placebo or usual care) are shown. Total enrolment by trial and treatment group are shown on the left; survival outcomes are included in the stacked bars (orange, patients who died; blue, patients who survived). The ORs and 95% CIs for mortality for each of the seven trials are shown on the right. The black vertical line represents an OR of 1·0 (no effect). RECOVERY enrolled more than twelve times as many patients as the five explanatory trials combined. Furthermore, none of the explanatory trials was sufficiently powered to demonstrate statistically significant results for mortality, and a meta-analysis combining all of the explanatory trials would still have failed to conclusively demonstrate that corticosteroids improve mortality in COVID-19. CAPE COVID=Community-Acquired Pneumonia: Evaluation of Corticosteroids.84 CoDEX=COVID-19-associated ARDS Treated With Dexamethasone: Alliance Covid-19 Brasil III.85 COVID STEROID=Hydrocortisone for COVID-19 and Severe Hypoxia.86 Steroids-SARI=Glucocorticoid Therapy for COVID-19 Critically Ill Patients With Severe Acute Respiratory Failure.86 DEXA-COVID19=Efficacy of Dexamethasone Treatment for Patients With ARDS Caused by COVID-19.87 OR=odds ratio. RECOVERY=Randomised Evaluation of COVID-19 Therapy.8, 31, 32 REMAP-CAP=Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia.88