Figure 9.
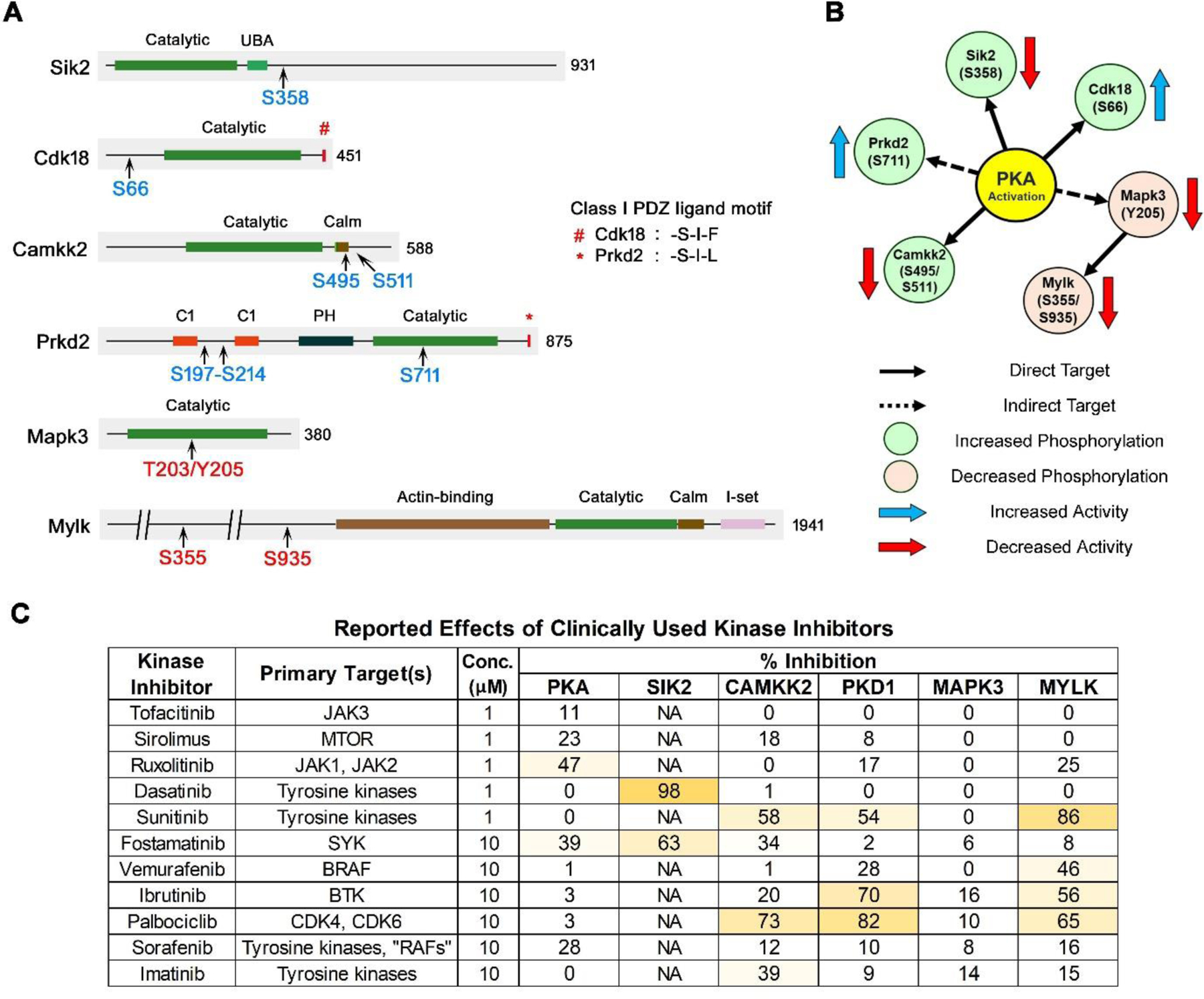
Protein kinases that undergo phosphorylation as either direct or indirect targets of PKA. A. Locations of vasopressin-responsive phosphorylation sites are indicated in blue (increased) or red (decreased). Class I PDZ ligand motif on the carboxyl terminal is shown. The total number of amino acid residues in each kinase is shown. Calm, calmodulin-binding domain; C1, conserved region-1/ diacylglycerol-binding domain; PH, plekstrin homology phosphoinositide binding domain; UBA, ubiquitin-associated domain; I-set, Immunoglobulin I-set domain. B. The relationship between PKA and downstream kinases are indicated using continuous (direct target) or dashed (indirect target) arrows. The direction of change in phosphorylation (blue, increased; red, decreased) and the resultant effect on the activity of the target kinase (blue arrow, increased; red arrow, decreased) are shown. Of note, increased phosphorylation can cause increase or decrease in the activity of a kinase and vice versa. C. Inhibition of protein kinases involved in vasopressin signaling by clinically utilized protein kinase inhibitors. Kinase inhibitor data were downloaded from the International Centre for Kinase Profiling (http://www.kinase-screen.mrc.ac.uk/kinase-inhibitors). Data for an expanded list of kinases are shown in Supplemental Table 7.
