Fig. 2. Nucleotide-binding pocket in the G-domain of IF2.
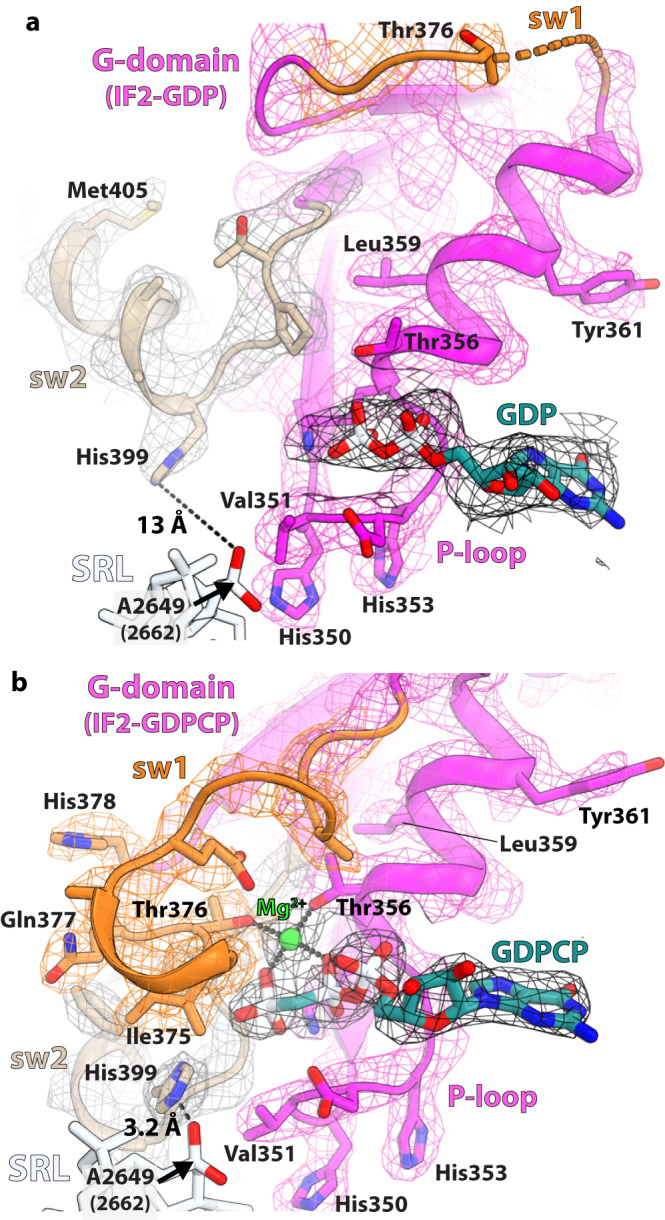
a The EM density of the GDP nucleotide and switch 2 (sw2) region in compact IF2 is shown as black and gray mesh, respectively, sw1 as orange mesh and the P-loop as pink mesh. The lack of density for sw1 (orange) indicates that it is disordered. The catalytic His399 residue in sw2 of IF2-GDP is oriented away from GDP and locates 13 Å from the phosphate oxygen of A2649 (E. coli A2662) in the SRL. b In extended IF2, the catalytic His399 is directed toward the γ-phosphate of GDPCP through the hydrophobic gate consisting of residues Val351 of the P-loop and Ile375 of sw1. In this position, His399 is positioned to interact with the phosphate oxygen of A2649 (A2662) in the SRL. Residues Thr376 of sw1 and Thr356 of the P-loop, together with the β- and γ-phosphate oxygen atoms of GDPCP, coordinate a magnesium ion (green).
