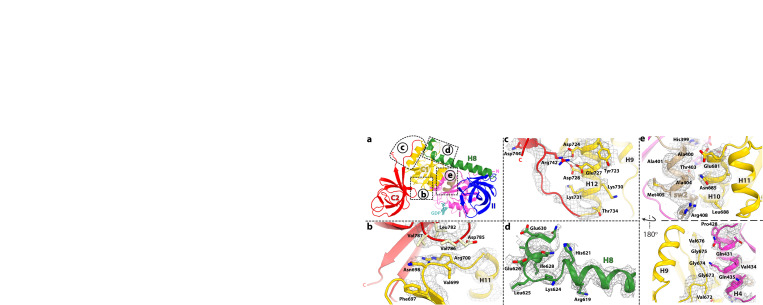
Fig. 4. Interactions between domains in compact IF2.
a Overview of IF2-GDP with inset boxes indicating close-up views. b Residues Asn698, Val699, and Arg700 in domain C1 (yellow) form a flat surface that stacks with β-hairpin residues Leu782, Asp785, Val786, and Val787 in domain C2 (red). EM density is shown as gray mesh. c Residues 735–742 form the linker between domains C1 (yellow) and C2 (red), which turns sharply by 180°. The last residue of the linker region (Arg742) interacts with the negative patch lining one side of helix H12 in domain C1. d EM density (gray mesh) of helix H8 (green) around residue Leu625. The 90°−turn of helix H8 is required to accommodate the new location of domain C1 in IF2-GDP. e Two views of the interface between the G-domain and domain C1. The β-strand Val672-(Gly)3-Val676 (lower panel) and helix H10 (upper panel) in domain C1 form the interface with H4 (lower panel) and the sw2-α-helix (upper panel) in the G-domain, respectively.