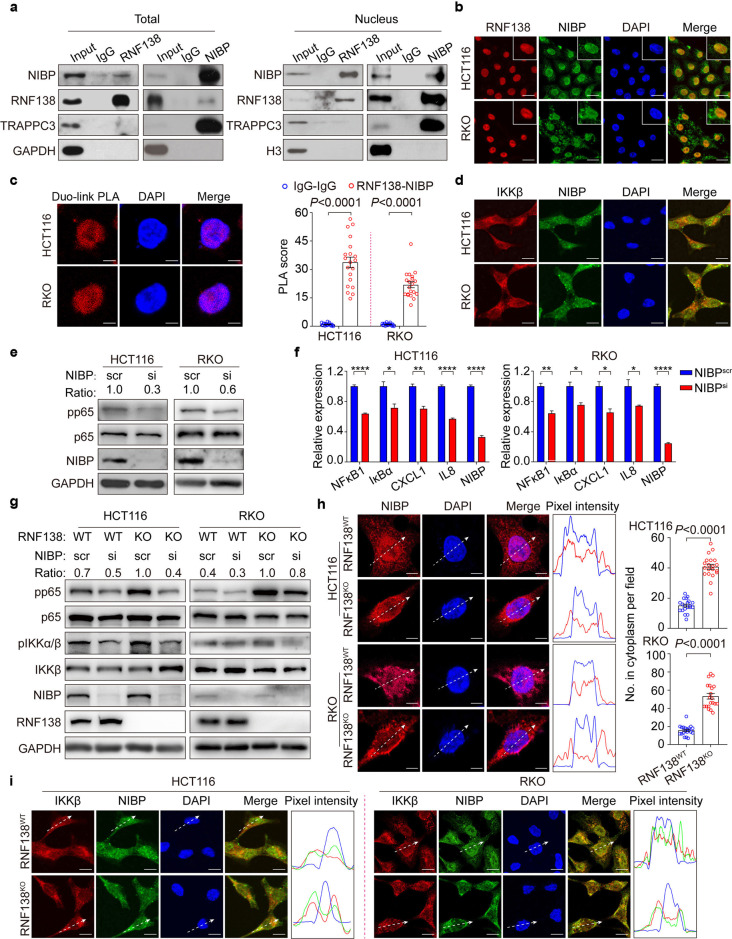
Fig. 4.
Nuclear-cytoplasmic partitioning of NIBP is regulated by RNF138 and contributes to the activation of NF-κB signaling. a Co-immunoprecipitation analysis of endogenous RNF138 and NIBP interactions in HCT116 total lysates (left) and nuclear extracts (right). IgG was used as control. b Immunostaining analysis of RNF138-NIBP co-localization in HCT116 and RKO cells. Nuclei were stained with DAPI. Insets show stained areas in more detail. Scale bar, 20 μm. c Representative confocal images (left) and quantification (right, n = 20) of endogenous RNF138 and NIBP interactions in HCT116 and RKO cells using proximity ligation assay. IgG were used as controls. Scale bar, 5 μm. d Immunostaining analysis of the co-localization of endogenous IKKβ and NIBP in HCT116 and RKO cells. Scale bar, 5 μm. e Immunoblots of phosphorylation, total levels of p65 in HCT116 and RKO cells transfected with si-NIBP or control siRNA. f qPCR analysis of NF-κB target gene expression in HCT116 and RKO cells (n = 3) with NIBP inhibition. *P < 0.05; **P < 0.01 and ****P < 0.0001. g Immunoblots of phosphorylation and total levels of p65 and IKKβ in RNF138WT and RNF138KO HCT116 and RKO cells with NIBP inhibition. h Representative immunostaining images of NIBP localization in RNF138WT and RNF138KO HCT116 and RKO cells, and the distribution of relative fluorescence intensities of lines scanned across the nucleus (right). Quantification of cytoplasmic NIBP intensity in HCT116 and RKO cells (n = 20). Scale bar, 5 μm. i Immunostaining analysis of IKKβ-NIBP co-localization in RNF138WT and RNF138KO HCT116 and RKO cells and relative pixel intensity of lines scanned across the nucleus. Scale bar, 20 μm