Abstract
Background
Osteomyelitis is inflammation of medullary cavities, haversian system and adjacent cortex of bone. It is devastating to patients when invasive.
Aim
The purpose of this study is to retrospectively review patients diagnosed with diabetic maxillary osteomyelitis and evaluate factors relating infection & diabetes.
Methodology
Case records of patients diagnosed with diabetic maxillary osteomyelitis were studied. Patient’s demographic data, predisposing factors, etiology, clinical features, culture sensitivity reports, microbiology, treatment and complications were studied. Diabetic status was confirmed by glycosylated hemoglobin (HbA1c) test. Duration of diabetes and anti-diabetic medication adherence was also studied.
Results
There were 28 patients diagnosed with diabetic maxillary osteomyelitis, (23—male; 5—female). Majority of the patients (60.7%) belonged to fourth & fifth decades. Twenty (71.4%) patients had poorly controlled diabetes (HbA1c > 8%). All patients reported with random blood sugar > 200 mg/dl. Thirteen patients (46.4%) were diagnosed for diabetes on admission and 11 patients (39.3%) had poor anti-diabetic medication adherence. Predominant etiology was odontogenic infection (50%). Cases of bacterial osteomyelitis (50%) were more frequent than those of fungal osteomyelitis (32.1%). Recurrence was observed in three cases.
Conclusion
Non-cognizance about diabetes mellitus can prove devastating for maxillofacial region and may prove fatal for the patient.
Keywords: Antibiotics, Diabetes, Infection, Maxilla, Osteomyelitis
Introduction
Osteomyelitis is defined as inflammation of bone which begins as infection of medullary cavity with rapid involvement of haversian systems and extension to periosteum [1]. During pre-antibiotic era, osteomyelitis of jaws was frequently encountered [2]. With advent of antibiotics and improved surgical treatment, there was marked reduction in incidence of this disease with improved prognosis. Recently, there appears to be a definite increase in prevalence of this condition due to increasing incidence of systemic diseases that compromise host immunity. These include uncontrolled diabetes mellitus (UDM), human immunodeficiency virus (HIV) infections, patients on immunosuppressive/chemo-therapy, malnutrition and those who have undergone radiotherapy [3–6].
Osteomyelitis is rarely seen in maxilla and also, maxilla rarely undergoes necrosis [7]. This is due to certain inherent features of maxilla such as rich vascularity, collateral blood flow, porous nature, scarcity of medullary tissues, thin cortices and presence of bone marrow with struts [5]. These features precludes confinement of infection within bone and permit dissipation of edema and pus into soft tissue and paranasal sinuses, thus hindering bacterial colonization. However, diminished host defences can alter clinical course of maxillary osteomyelitis and may cause serious complications such as infection of cranial cavity [8]. An incidence of 61%–68% cases of maxillary osteomyelitis are related to diabetes mellitus [3, 9]. An association of maxillary osteomyelitis with diabetes mellitus can be termed as Diabetic Maxillary Osteomyelitis (DMO) and can be defined as a condition of maxilla that occurs specifically in patients with diabetes mellitus (HbA1c > 5.7%) leading to osteomyelitis and necrosis of maxilla.
There is a paucity of studies done on maxillary osteomyelitis and its correlation with diabetes. Hence, we conducted study with the aim to investigate and determine various factors relating maxillary osteomyelitis and diabetic status.
Materials and Methods
Case records of patients with DMO treated during the 5-year period from July 2014 to June 2019 were studied. Ethical approval was obtained from institutional review board. Inclusion and exclusion criteria are as follows:
Inclusion Criteria
Proven history of diabetes
Newly diagnosed / undiagnosed diabetes
Post-trauma cases with diabetes
Glycosylated hemoglobin (HbA1c) ≥ 5.7%
Exclusion Criteria
No history of diabetes with blood sugar levels within normal limits
Post-radiotherapy
HIV infections
Immunosupressive/chemo-therapy
Standard data collection included age, gender, chief complaint, history of present illness, medical history, blood sugar levels, clinical presentation, probable etiology, histopathology, culture sensitivity reports, treatment performed, follow-up complications, recurrence and rehabilitation. Diagnosis of DMO was based on history and clinico-radio-histological findings. Diabetic status of patient at time of reporting/admission, duration of diabetes mellitus, anti-diabetic medication adherence, possible etiology and microbiology of the disease, and efficacy of treatment protocol were studied. Diabetic status of the patient was checked by investigating blood sugar profile which included HbA1c and random blood sugar level (RBSL).
Treatment Protocol Followed
Broad spectrum empirical antibiotic therapy was initiated on admission.
Constant monitoring & correction of the raised blood sugar levels within normal limits.
Radiographic investigations—Computed tomographic scan (CT scan) / Cone beam computed tomogram (CBCT)—were performed to assess the extent of the disease.
Incisional biopsy for histopathological diagnosis and culture sensitivity
Definitive surgical treatment
Administration of culture guided antibiotics / antifungal agents if empirical antibiotics were clinically ineffective.
Prosthetic rehabilitation
Results
Data collected in our study are summarized in Table 1. Out of 28 patients, 23 patients were male & 5 patients were female (M:F 4.6:1). Age of patients ranged from 21 to 72 years with mean age of 50.5 years. Maximum patients (60.7%) belonged to fourth & fifth decades of life. At time of admission, 71.4% patients had HbA1c > 8% i.e., were cases of uncontrolled diabetes and all patients (100%) had RBSL > 200 mg/dl. Thirteen (46.4%) patients were unaware about their diabetic status and 11 (39.3%) patients had poor anti-diabetic medication adherence. The most common etiology was odontogenic infections (50%) followed by maxillary sinusitis (28.6%), and tooth extraction (53.6%) was most common predisposing factor. Fourteen (50%) of the positive cultures showed infections of mixed bacterial flora while fungal growth was evident in nine patients (32.1%). Aspergillosis & mucormycosis were predominant variants of fungal osteomyelitis. No growth was observed in five cases. Two patients were not able to continue treatment due to financial constraints. Two patients were referred for hyperbaric oxygen therapy (HBOT); however, both patients did not report back and were lost on follow-up. Rest 24 patients were started with a course of empirical antibiotic therapy. Once the blood sugar levels were controlled, surgery was performed (Fig. 1). Surgical debridement and curettage was performed until bone starts bleeding and lesion-free bony borders were clinically verified. Local tissue advancement (11 patients), buccal pad of fat (6 patients), buccal myomucosal flap (3 patients), temporalis myofacial flap (2 patients) and maxillary feeding plate (2 patients) were used to close the maxillary defect.
Table 1.
Data collected
| Pati- ent | Age (years) | Gender | HbA1c (%) | RBSL (mg/dl) | Duration of DM (years) | Anti-diabetic Medication Adherence | Etiology | Predisposing factor | Microbiology/Histopathology |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 48 | M | 9.78 | 387 | Undiagnosed | – | Odontogenic | Extraction | Gram –ve cocci |
| 2 | 46 | M | 9.34 | 354 | Undiagnosed | – | Sinusitis | Extraction | Enterococci |
| 3 | 55 | M | 12.42 | 607 | 15 | Nil | Sinusitis | Common cold | Mucormycosis |
| 4 | 70 | F | 10.56 | 520 | 30 | Irregular | Rhinitis | Common cold | Mucormycosis |
| 5 | 21 | M | 8.54 | 306 | Undiagnosed | – | Odontogenic | Extraction | Mucormycosis |
| 6 | 65 | M | 6.37 | 219 | 12 | Insulin (Regular) | Rhinitis | Common cold | Pseudomonas |
| 7 | 35 | M | 8.66 | 266 | Undiagnosed | – | Odontogenic | Extraction | No Growth |
| 8 | 60 | M | 8.22 | 297 | 3 | OHA (Regular) | Odontogenic | Extraction | No Growth |
| 9 | 55 | M | 10.46 | 435 | 12 | Irregular | Odontogenic | Extraction | Actinomycosis |
| 10 | 60 | M | 11 | 478 | Undiagnosed | – | Odontogenic | Extraction | Aspergillosis |
| 11 | 55 | M | 8.18 | 260 | 8 | Irregular | Odontogenic | Extraction | Enterococci |
| 12 | 57 | M | 8.58 | 286 | Undiagnosed | – | Odontogenic | Extraction | No Growth |
| 13 | 57 | M | 8.34 | 312 | 1 | Irregular | Sinusitis | Recurrence | No Growth |
| 14 | 42 | M | 8.68 | 298 | Undiagnosed | – | Odontogenic | Extraction | No Growth |
| 15 | 45 | M | 8.46 | 242 | Undiagnosed | – | Sinusitis | Perio. Therapy | Enterococci |
| 16 | 46 | F | 7.38 | 238 | 3 | Irregular | Unknown | Unknown | Actinomycosis |
| 17 | 70 | M | 10.22 | 458 | 18 | Nil | Odontogenic | Extraction | Aspergillosis |
| 18 | 40 | M | 8.84 | 296 | 3 | Nil | Sinusitis | Common cold | Aspergillosis |
| 19 | 36 | F | 6.98 | 236 | Undiagnosed | – | Unknown | Unknown | Aspergillosis |
| 20 | 32 | M | 8.42 | 245 | Undiagnosed | – | Odontogenic | Perio. therapy | Aspergillosis |
| 21 | 42 | M | 6.45 | 216 | 4 | Insulin (Regular) | Odontogenic | Extraction | Staphylococcus |
| 22 | 40 | F | 6.87 | 226 | Undiagnosed | – | Sinusitis | Common cold | Gram –ve cocci |
| 23 | 49 | M | 7.68 | 224 | 4 | OHA (Regular) | Sinusitis | Extraction | Pseudomonas |
| 24 | 50 | M | 10.24 | 473 | Undiagnosed | – | Trauma | Trauma | Staphylococcus |
| 25 | 72 | M | 10.86 | 396 | 22 | Nil | Odontogenic | Extraction | Mucormycosis |
| 26 | 54 | M | 7.45 | 235 | 5 | Irregular | Sinusitis | Common cold | Diptheroids |
| 27 | 64 | M | 9.65 | 324 | 13 | Nil | Odontogenic | Extraction | Diptheriods |
| 28 | 49 | F | 7.86 | 236 | Undiagnosed | – | Trauma | Trauma | Actinomycosis |
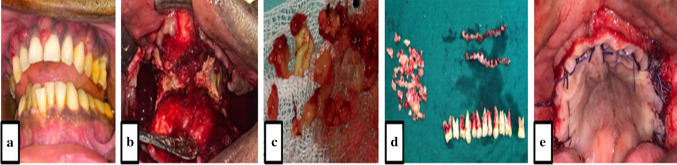
Fig. 1.
Definitive surgical treatment. a Typical small abscesses involving whole maxilla. b Sequestrectomy done. c Infected soft tissue and maxillary sinus lining. d Necrotic bone tissue and extracted teeth. e Primary closure by advancement of local tissue
Postoperative care consisted of continuing with antibiotic treatment, intra-oral irrigations & dressings. Culture guided antibiotics were administered, if empirical antibiotics were ineffective. The mean duration of intravenous (IV) postoperative antibiotics was 10 days, after that, patients were shifted to oral antibiotics for 3 weeks. In patients who were diagnosed with fungal osteomyelitis, additional antifungal protocol was followed. Patients were discharged on satisfactory recovery and healing. All patients were recalled for follow-up after 1 week, 2 weeks and every month for next 6 months. Wound healing was satisfactory with no evidence of complications in 13 patients (54.2%). Eleven patients (45.8%) had postoperative complications which included fistula formation, wound dehiscence, paresthesia & recurrence. Seven patients were lost on long-term follow-up. After complete resolution of disease, prosthetic rehabilitation was done with obturator cum denture prosthesis.
Certain striking clinical features in our study include:
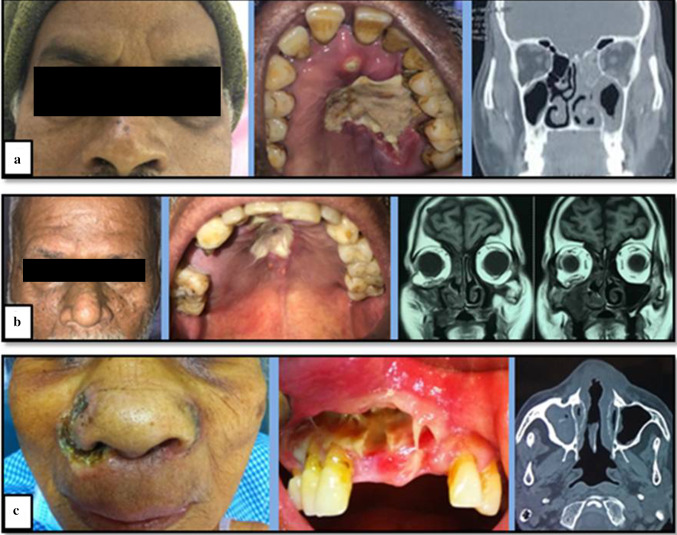
One patient (case of Mucormycosis) reported with ptosis and loss of vision in left eye (Fig. 2a). Patient was in disorientated & confused state with deranged renal function tests. Features were suggestive of cavernous sinus involvement.
Another patient (case of Aspergillosis) had extension of infection till orbital floor leading to pathological fracture of orbital floor & subsequently enopthalmos of right eye (Fig. 2b).
Another patient (case of Mucormycosis) reported with perforating ulcerative cutaneous lesion extra-orally (Fig. 2c).
Hard palate and both maxilla were completely necrosed and exposed revealing extensive involvement in a case of Aspergillosis.
Sixteen patients had palatal bone involvement also.
In eight patients, ethmoid & sphenoid sinuses were also involved.
Fig. 2.
Striking clinical presentation. a Case of mucormycosis with ptosis and loss of vision in left eye. b Case of Aspergillosis with extension of infection till orbital floor leading to enopthalmos of right eye. c Case of Mucormycosis with perforating ulcerative cutaneous lesion extra-orally
Discussion
The occurrence, type, severity, management and prognosis of osteomyelitis depend on microbiota, host immunologic response, source and extension of infection. There may be presence of infection within jaws or within tooth/teeth, or pathogens are introduced through exposed mucosa [10]. Entry of microbes into cancellous bone causes vascular compression leading to ischemia and avascular necrosis of bone. Immobile and stagnant blood also acts as nidus for development of infection [11]. Compromised host defence significantly increases susceptibility to infection [4, 10]. Progressive bone destruction and sequestrum formation are characteristic feature [8].
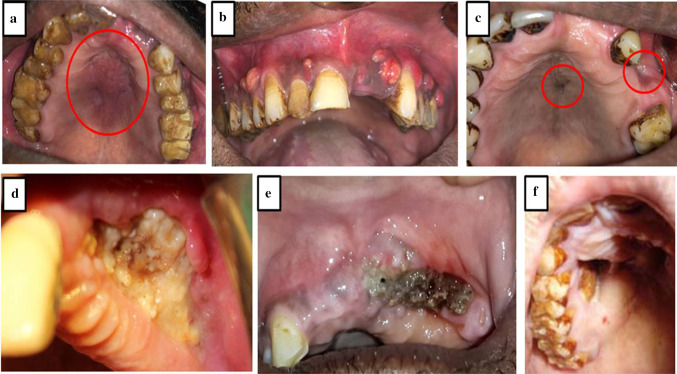
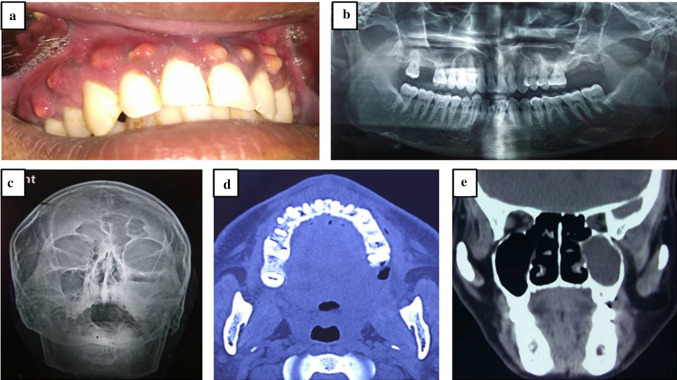
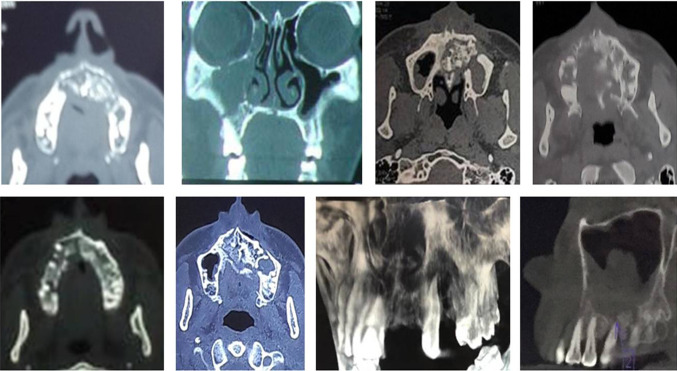
Typical clinical presentation included mobile maxilla, multiple mobile and tender teeth (Fig. 2a, b), tender swelling (Fig. 3a), purulent discharge, multiple small abscesses (Fig. 3b), oro-antral fistula/oro-nasal fistula (Fig. 3c), skin fistula (Fig. 2c), exposed necrotic bone with non healing soft tissue (Fig. 3d, e, f), trismus, paresthesia and localized intense pain in the involved region. Majority of the patients had a definite odontogenic etiology (50%) followed by maxillary sinusitis (28.6%). Other etiological factors included trauma (7.2%) and rhinitis (7.2%). In two patients (7.2%), no definite cause could be identified. The dental infection can arise either from a root canal, periodontal ligament, fracture site, soft tissue wound or surgical site like extraction socket [12]. Certain factors predisposed diabetic patients to maxillary osteomyelitis. These factors include tooth/teeth extraction, common cold, periodontal therapy and trauma to maxillofacial region (Table 1). In two cases, we were not able to ascertain the predisposing factor which suggests that the disease may have idiopathic nature also. The male-to-female (M:F) ratio was found to be 4.6:1. In the literature, M:F ratio has been reported to vary from 2.1:1 to 5.2:1 [3, 13]. Biopsy was done essentially to confirm diagnosis and rule out any neoplastic lesion. Radiographically, maxillary osteomyelitis appears as a radiolucent lesion with sequestra formation. Lesions are usually large with undistinguishable borders [14]. We performed conventional radiographs (orthopantamogram & paranasal sinus views) and CBCT/CT scan (Fig. 4a–e) to assess extent of the disease and to plan surgical procedure. Magnetic resonance imaging (MRI) was advised in case of extension of infection to orbital cavity (Fig. 2b). Radiographic and tomographic study revealed involvement of alveolar bone, maxilla, hard palate, zygomatic bone and adjoining areas. There was extensive bone loss, presence of sequestra and extension upto maxillary sinus, nasal cavity, pterygoid plates, sphenoid sinus and ethmoid sinuses (Figs. 2, 3, 5).
Fig. 3.
Typical clinical presentation of maxillary osteomyelitis. a Palatal swelling. b Multiple small abscesses. c Oro-antral fistula/oro-nasal fistula. d–f Exposed necrotic bone with non-healing soft tissue
Fig. 4.
Radiographic investigations. a Typical small abscesses involving whole maxilla. b Orthopantamogram. c Paranasal sinus view. d CT scan (axial view). e CT scan (coronal view)
Fig. 5.
Varying tomographic presentation of maxillary osteomyelitis
Both bacterial and fungal microbiology were evident in our study (Table 1). Fourteen (50%) of positive cultures showed infections of mixed bacterial flora while fungal growth was evident in 9 patients (32.1%). Aspergillosis & mucormycosis were predominant variants of fungal osteomyelitis. No growth was observed in 5 (17.9%) patients and this might be due to previous treatment (antibiotics) started elsewhere. Coviello et al. reported that 93% of chronic osteomyelitis cases are polymicrobial, with an average of 3.9 organisms per specimen [10]. Maxillary osteomyelitis occurs due to polymicrobial bacteria such as bacteroides, peptostreptococcus and microaerophilic streptococcus along with opportunistic pathogens [8]. Fungal osteomyelitis is highly invasive, rapidly progressive, opportunistic and life-threatening disease of the maxillofacial region [15, 16]. Most common organism causing fungal infections is aspergillus (69.7%) followed by candida (22.2%) and zygomycetes (Mucorales) (8.1%) [9]. According to Anehosur et al., incidence of fungal maxillary osteomyelitis is 52% with M:F ratio being 2.1:1 and age group between 10–65 years [17]. Niranjan et al. observed 52% cases of fungal osteomyelitis and 48% of nonfungal osteomyelitis in a 10-year study. Fungal osteomyelitis was frequently found above 40 years of age (80.76%), more common in males (69.23%) and affecting maxilla (80.76%), anterior maxilla affected more commonly than posterior. Fungal osteomyelitis was more commonly associated with diabetes mellitus (61.53%) [9]. In our study, nine cases (32.2%) of DMO had fungal etiology and aspergillosis was more common (5 cases—55.5%) than mucormycosis (4 cases—45.5%). Also, it was frequently found above 40 years of age (66.7%), more common in males (77.8%) with M:F ratio being 3.5:1. Fungal microorganisms frequently colonize oral mucosa, nasal mucosa, paranasal sinuses and pharyngeal mucosa in asymptomatic patients and are usually avirulent; they become pathogenic only when host resistance is exceptionally low [17]. Diabetes mellitus is the most common (60–81%) predisposing factor [9]. Extraction socket is also an invasive portal site due to extraneous contamination [15–18]. In orofacial region, clinical manifestation of fungal infection includes rhinorrhea, facial cellulitis, nasal discharge and turbinate necrosis. Ophthalmic involvement includes painful eyes, blurred vision, conjunctival suffusion, ptosis, proptosis, chemosis and loss of vision due to retinal artery thrombosis [15, 19]. Maxilla, hard palate, paranasal sinuses, alveolar mucosa and buccal mucosa are affected intraorally [18]. Rhino-orbito-cerebral involvement is the most severe and life-threatening and can result in cerebral ischemia and death [9, 15–17]
Kim et al. reported 94.9% success rate when surgery is followed by 2 weeks of IV antibiotics (augmentin, cefazolin and aminoglycoside) followed by 6 weeks of oral administration (augmentin and roxythromycin). Clindamycin and metronidazole used according to culturing and sensitivity tests [20]. Empirical broad spectrum antibiotics were started for all patients. In our study, main drug of antibiotic regimen was β-lactams. Clindamycin was administered to patients allergic to β-lactams. Metronidazole was administered to treat anaerobic flora. Gentamycin was added for gram negative infection like pseudomonas. Duration of postoperative antibiotics depended on response of patients to antibiotic therapy. Eighteen patients (64.3%) recovered within 2—3 weeks of antibiotic therapy. We recommend duration of antibiotic course for 6 weeks, typically beginning with 2 weeks of intravenous antibiotics followed by a 4 weeks of oral antibiotics. Beta-lactam, Clindamycin and Metronidazole form mainstay of antimicrobial treatment. Treatment of fungal osteomyelitis requires additional anti-fungal treatment protocol. Due to cost factor & low socio-economic status of our patients in rural region, Amphotericin B regimen could not be followed in our study. We administered 400 mg Fluconazole (6 mg/kg) IV once daily for one week postoperatively followed by oral administration with tablet fluconazole 400 mg/day for 1 month.
Surgical intervention is aimed at providing drainage to area of infection, removal of sequestrum and other foreign bodies and restoring new blood supply to affected region. It includes extraction of involved teeth, sequestrectomy, debridement, curettage of granulation tissue and meticulous closure of surgical defect. Removal of involved teeth is advocated as retained teeth pose risk of re-infection [21]. Sequestrectomy of necrotic maxilla and excision of infected mucosa should be performed for complete disease clearance. Surgical debridement and curettage should be performed until bone starts bleeding and lesion-free bony borders are clinically verified. Primary closure can be achieved by advancement of local tissue. Reconstruction of maxillary defects can be performed using buccal fat pad, temporalis muscle flap, buccal myomucosal flap, tongue flap or obturator prosthesis. On follow-up, all patients showed satisfactory resolution by about 8 weeks. Complication rate was 39.3% in our study. Three patients with oro-antral fistula underwent revision surgery using buccal pad of fat & buccal myomucosal flap. Two patients with wound dehiscence were managed conservatively with local debridement. Complete healing was achieved by secondary intention. Three patients complained of post-surgical paresthesia. They were reassured and counselled regarding recovery and kept under observation. Three patients (10.7%) had recurrence and secondary correction was performed after 6 months. All three recurred cases were previously diagnosed as fungal DMO. The relapse rate can be as high as 20% [8].
Osteomyelitis of the jaws is one of the serious maxillofacial complications of diabetes mellitus [9]. Diabetic status & incidence of maxillary osteomyelitis has a very strong co-relation among our diabetic patients. Only 2 (7.2%) patients diagnosed with diabetes mellitus were under good control (HbA1c ≤ 6.4%) & rest 26 patients (92.8%) had moderate to poor control blood sugar levels. At time of admission, 71.4% patients had HbA1c > 8%, i.e., were cases of UDM and all patients (100%) had RBSL > 200 mg/dl. Thirteen (46.4%) patients were unaware about their diabetic status and 11 (39.3%) patients had poor anti-diabetic medication adherence. Hence, we can conclude that 85.7% patients were non-cognizant about diabetes mellitus. In diabetes mellitus, primary contributing factors are deranged granulocyte-phagocytic ability and diminished polymorphonuclear leukocyte chemotaxis that permits innocuous microorganisms to proliferate. Second contributing factors include microangiopathy and atherosclerosis, which result in diminished vascularity & local tissue ischemia, thus reducing tissue perfusion and increased vulnerability to infection [15, 17]. Also, defective glucose utilization causes delayed wound healing [6]. High glucose levels, acidic environment, low oxygen and high iron levels facilitate germination and aggressive invasive growth of acquired fungal spores [15]. Excessive glycosylation of proteins (transferrin and ferritin) owing to hyperglycemia results in decreased affinity of these proteins to bind iron. Fungal hyphae produce “rhizoferrin” which binds to available serum iron and forms iron-rhizoferin complexes. This is the nutrient element required for growth, development, and multiplication of fungal spores [17]. A favorable environment is also created due to an excess of ketone bodies in diabetic patients. Rhizopus arrhizus produces the enzyme ketoreductase, which allows them to utilize the patient’s ketone bodies [22]. Elevated glucose and iron levels upregulate GRP78 expression and promote endothelial cell invasion and damage by fungus in a receptor dependent manner [9, 23, 24]. Vascular invasion is the key pathophysiological feature [18]. Spores or vegetative forms invade arteries and form thrombus within these vessels resulting in ischemic infarcts and subsequently necrosis of regional hard and soft tissues [25].
Diabetes mellitus is a silent killer and maxillary osteomyelitis should be taken into serious consideration in diabetic individuals, whether controlled or uncontrolled. Timely treatment is paramount to achieve total resolution of disease and reduce morbidity and mortality. Strict glycaemic control routinely as well as pre-, intra- & postoperative glycaemic control is mandatory to prevent as well as treat DMO. Empirical and culture-guided antibiotic & antifungal regimen should be followed strictly. Surgery forms mainstay for definitive cure. It is mandatory to remove any residual infected / necrotic bony or soft tissue to prevent recurrence.
Conclusion
There is definitely a need to create awareness that non-cognizance for diabetes can be dreadful and devastating for maxillaofacial region and can prove fatal. Do not let denial put patient’s health and life at risk. It is time to shift the paradigm from worrisome vulnerability to hopeful optimism.
Funding
Nil.
Compliance With Ethical Standards
Conflict of Interest
Authors declare that they have no conflict of interest.
Research Involving Human Participants
Yes (Retrospective study)
Informed Consent
Not applicable (As study is retrospective in nature)
Ethical Approval
The manuscript has been read and approved by all the authors, the requirements for authorship have been met, and each author believes that the manuscript represents honest work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Topazian RG, Goldberg MH, Hupp JR. Oral and maxillofacial infections. 4. Philadelphia: Saunders; 2002. Osteomyelitis of the jaws; pp. 214–235. [Google Scholar]
- 2.Reddy S, Prasad K, Chippagiri P, et al. Osteomyelitis of the maxilla: a case report of three cases. Am J Adv Med Sci. 2014;2(3):34–41. [Google Scholar]
- 3.Peravali RK, Jayade B, Joshi A, Shirganvi M, Rao CB, Gopalkrishnan K. Osteomyelitis of maxilla in poorly controlled diabetics in a rural Indian population. J Maxillofac Oral Surg. 2012;11(1):57–66. doi: 10.1007/s12663-011-0283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel V, Harwood A, McGurk M. Osteomyelitis presenting in two patients: a challenging disease to manage. Br Dent J. 2010;209:393–396. doi: 10.1038/sj.bdj.2010.927. [DOI] [PubMed] [Google Scholar]
- 5.Hudson JW. Osteomyelitis of Jaws: a 50-year perspective. J Oral Maxillofac Surg. 1993;51:1294–1301. doi: 10.1016/S0278-2391(10)80131-4. [DOI] [PubMed] [Google Scholar]
- 6.Yadav S, Malik S, Mittal HC, Puri P. Chronic suppurative osteomyelitis of posterior maxilla: a rare presentation. J Oral Maxillofac Pathol. 2014;18:481. doi: 10.4103/0973-029X.151364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baltensperger M, Gratz K, Bruder E, Lebeda R, Makek M, Eyrich G. Is Primary chronic osteomyelitis a uniform disease? Proposal of a classification based on a retrospective analysis of patients treated in the past 30 years. J Cranio-Maxillofac Surg. 2004;32:43–50. doi: 10.1016/j.jcms.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Singh M, Singh S, Jain J, Singh KT. Chronic suppurative osteomyelitis of maxilla mimicking actinomycotic osteomyelitis: a rare case report. Nat J Maxillofac Surg. 2010;1:153–156. doi: 10.4103/0975-5950.79219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niranjan KC, Sarathy N, Alrani D, Hallekeri K. Prevalence of fungal osteomyelitis of the jaws associated with diabetes mellitus in North Karnataka population: a retrospective study. Int J Cur Res. 2016;8:27705–27710. [Google Scholar]
- 10.Coviello V, Stevens MR. Contemporary concepts in the treatment of chronic osteomyelitis. Oral Maxillofac Surg Clin North Am. 2007;19:523–534. doi: 10.1016/j.coms.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Strumas N, Antonyshyn O, Caldwell CB, Mainprize J. Multimodality imaging for precise localization of craniofacial osteomyelitis. J Craniofacial Surg. 2003;14(2):215–219. doi: 10.1097/00001665-200303000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Koorbusch GF, Fotos P, Goll KT. Retrospective assessment of osteomyelitis, etiology, demographics, risk factors and management in 35 cases. Oral Surg Oral Med Oral Pathol. 1992;74:149–154. doi: 10.1016/0030-4220(92)90373-X. [DOI] [PubMed] [Google Scholar]
- 13.Rangne A, Rudd A. Osteomyelitis of the jaws. Int J Oral Surg. 1978;7:523–527. doi: 10.1016/S0300-9785(78)80068-4. [DOI] [PubMed] [Google Scholar]
- 14.Marx R, Stern D. Oral and maxillofacial pathology. A rationale of diagnosis and treatment, 1st Ed, Quintessence, 2003.
- 15.Srivastava A, Mohpatra M, Mahapatra A. Maxillary fungal osteomyelitis: a review of literature and report of a rare case. Ann Maxillofac Surg. 2019;9:168–173. doi: 10.4103/ams.ams_218_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma A, Singh V, Jindal N, Yadav S. Necrosis of maxilla, nasal, and frontal bone secondary to extensive rhino-cerebral mucormycosis. Natl J Maxillofac Surg. 2013;4:249–251. doi: 10.4103/0975-5950.127663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anehosur V, Agrawal SM, Joshi VK, Anand J, Krishnamuthy K, Kumar N. Incidence and treatment protocol for maxillofacial fungal osteomyelitis: a 12-year study. J Oral Maxillofac Surg. 2019;77:2285–2291. doi: 10.1016/j.joms.2019.06.187. [DOI] [PubMed] [Google Scholar]
- 18.Afroze SN, Korlepara R, Rao GV, Madala J. Mucormycosis in a diabetic patient: a case report with an insight into its pathophysiology. Contemp Clin Dent. 2017;8:662–666. doi: 10.4103/ccd.ccd_558_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harril WC, Stewart MG, Lee AG, Cernoch P. Chronic rhinocerebral mucormycosis. Laryngoscope. 1996;106:1292–1297. doi: 10.1097/00005537-199610000-00024. [DOI] [PubMed] [Google Scholar]
- 20.Kim SG, Jang HS. Treatment of chronic osteomyelitis in Korea. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:394–398. doi: 10.1067/moe.2001.117810. [DOI] [PubMed] [Google Scholar]
- 21.Bevin CR, Inwards CY, Keller EE. Surgical management of primary chronic osteomyelitis: a long-term retrospective analysis. J Oral Maxillofac Surg. 2008;66:2073–2085. doi: 10.1016/j.joms.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 22.Urs AB, Singh H, Mohanty S, Sharma P. Fungal osteomyelitis of maxillofacial bones: Rare presentation. J Oral Maxillofac Pathol. 2016;20:546–547. doi: 10.4103/0973-029X.190966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrahim AS, Kontoyiannis DP. Update on mucormycosis pathogenesis. Curr Opin Infect Dis. 2013;26(6):508–515. doi: 10.1097/QCO.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(S1):S16–22. doi: 10.1093/cid/cir865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fogarty C, Regennitter F, Viozzi CF. Invasive fungal infection of the maxilla following dental extractions in a patient with chronic obstructive pulmonary disease. J Can Dent Assoc. 2006;72:149–152. [PubMed] [Google Scholar]