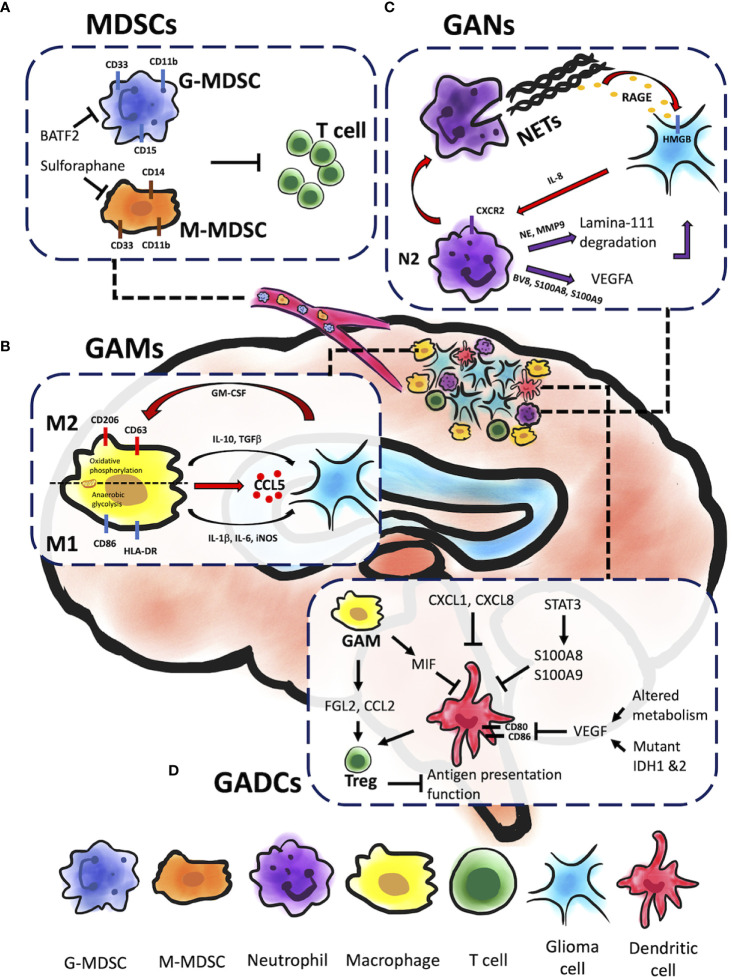
Figure 1.
Myeloid cells within glioma microenvironment. Gliomas are composed of different types of myeloid immune cells which promote tumor progression, including MDSCs, GAMs, GANs, and GADCs. Each of these cell types contributes to glioma progression in unique ways. (A) Both G-MDSC and M-MDSC recruitments contribute to T cell inactivation and inhibition cytotoxicity of glioma cells. BATF-2 on G-MDSC and sulforaphane on M-MDSC could cause inhibitory effect and further prevent from T cell inactivation and glioma progression. (B) GAMs engage in significant bidirectional crosstalk with glioma cells. Glioma cells release cytokines and chemoattractants to recruit GAMs to the glioma microenvironment, and M2 GAMs in turn supply pro-tumorigenic and pro-survival factors. In addition, GM-CSF promote GAMs’ mitochondrial reprograming that sway between M1 and M2 inflammatory response leading to glioma resistance. (C) GANs can be reprogrammed to express pro-tumor phenotype (N2) with TGFβ signaling in the TME to facilitate tumor growth through NE and MMP9 secretion. The release of the pro-angiogenic factors BV8 and the S100 proteins (S100A8 and S100A9) by N2 GANs activate VEGFA to promote tumor growth. Glioma cells can induce NETs formation via IL-8 production. NETs are correlated with glioma progression and prognosis through a HMGB1/RAGE/IL-8 axis. (D) A variety of signaling molecules alter GADC migration, infiltration of the TME, maturation, and function. FGL2 and CCL2 secreted by GAMs and GADCs induce Treg activity, which suppresses antigen presentation function of GADCs. MIF, also secreted by GAMs, inhibits GADC maturation as well as migration and infiltration to the TME. The STAT3 signaling pathway inhibits GADC maturation, as does VEGF through inhibition of costimulatory factors CD80 and CD86. VEGF is expressed by tumor cells and influenced by mutant IDH1 and IDH2, as well as altered metabolism in the TME.