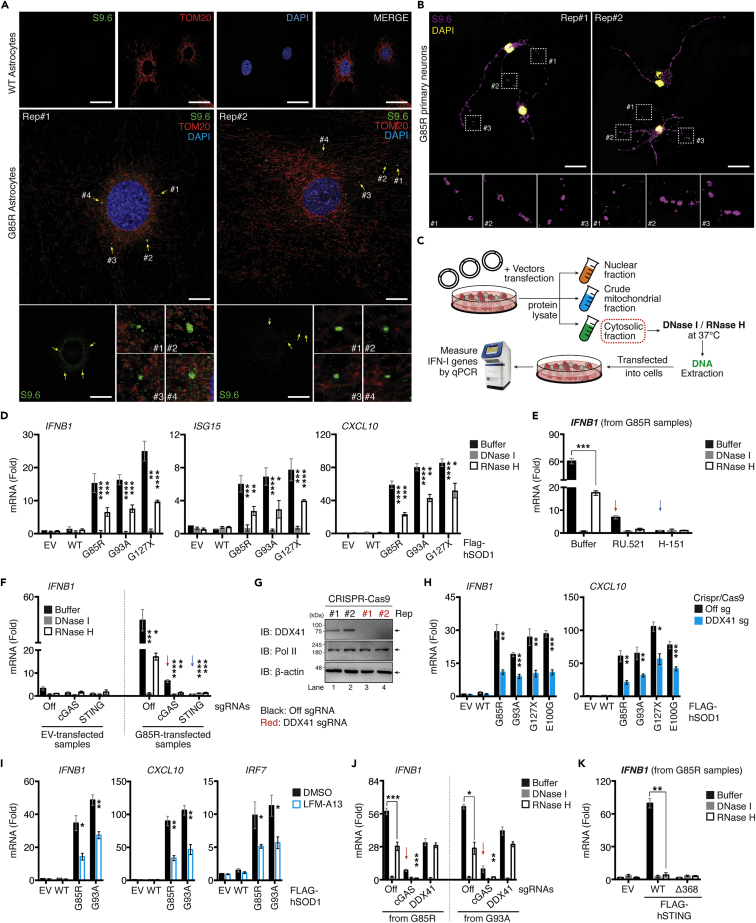
Figure 6.
ALS SOD1-mutants trigger mt(RNA:DNA) hybrid release and activate IFN-I responses through the DDX41-STING axis
(A and B) Primary neurons and astrocytes extracted from WT or G85R-mice at day-10 after birth were fixed and immunostained with indicated antibodies and DAPI (blue).
(C) Schematic diagram of DNase I/RNase H treatment experiments. Pure cytosolic fraction from C20 cells transfected with 0.01 μg of Flag-SOD1 WT or SOD1-mutant vectors for 36 h were incubated with either buffer, DNase I or RNase H, followed by DNA extraction. These samples were re-transfected into cells and subjected to gene quantification.
(D) RT-qPCR analysis of the indicated mRNA from samples derived from experimental design described in panel C.
(E) WT C20 cells treated with RU.521 (5.0 μg/mL) or H-151 (0.5 μg/mL) for 24 h. Cells were then transfected with DNA extracted from EV and G85R-transfected samples as described in C. RNA was purified and Ifnb1 mRNA was measured using RT-qPCR.
(F) WT, cGAS, or STING-deficient C20 cells were transfected with DNA extracted as described in panel C, Ifnb1 mRNA was measured via RT-qPCR.
(G) CRISPR/Cas9 DDX41-deficient C20 cells validated by western blotting.
(H) WT or DDX41-deficient C20 cells were transfected with 0.01 μg of indicated plasmids, and the indicated mRNA was measured using RT-qPCR.
(I) WT C20 cells transfected with the indicated plasmids and treated with DMSO or LFM-A13 (100 μM). RNA was purified and Ifnb1 mRNA was measured via RT-qPCR.
(J) DNA extracted from G85R or G93A-transfected samples and prepared as described in panel C were re-transfected into WT, cGAS, or DDX41-deficient cells for 6 h. Ifnb1 mRNA expression was measured.
(K) DNA extracted from G85R-transfected samples prepared as described in C were re-transfected into HEK293T cells expressing either EV, Flag-tagged STING-WT, or STING Δ368. RNA was purified and Ifnb1 mRNA was measured using RT-qPCR. All RT-qPCR of RNA samples was normalized to β-actin. Data represent the mean of three independent experiments (mean ± SEM). White scale bars correspond to 10 μm ∗p< 0.05, ∗∗p< 0.01, ∗∗∗p< 0.001, ∗∗∗∗p< 0.0001; (D–F and H–K: unpaired Student’s t test). See also Figure S6.