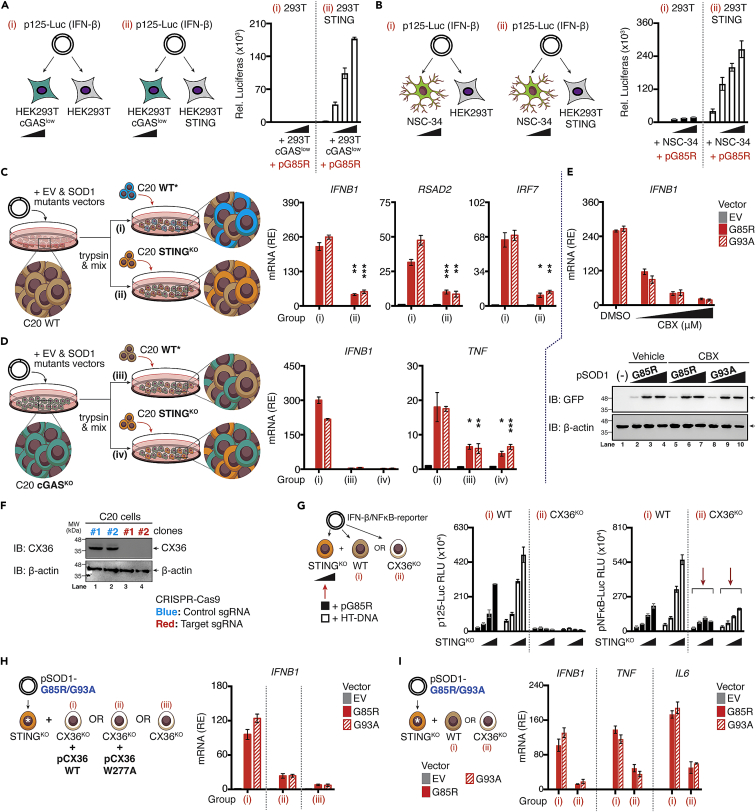
Figure 7.
Connexin-36 mediate cGAMP(2′ 5′) intercellular transfer in human microglia
(A and B) HEK293T and HEK293T-STING cells were co-cultured with HEK293T cGASlow or NSC-34 cells (ratio ranging from 1:0.5 to 1:0.125), followed by transfection with p125-Gluc (IFN-β), whereas transactivation of the reporter was assessed after 24 h.
(C and D) WT (C, brown), or cGAS-deficient C20 cells (D, green) expressing either EV, SOD1-WT, or mutants were co-cultured with WT [marked by asterisk (∗), blue] or STING-deficient C20 cells (orange). Relative induction of indicated mRNA from each group (i, ii, iii, or iv) was measured via RT-qPCR.
(E) C20 cells expressing EV, WT, or mutants SOD1 in the absence or presence of CBX (150 μM) were subjected to RT-qPCR of Ifnb1 mRNA (upper). The protein level of indicated SOD1 mutants in C20 cells after treatment with CBX was assessed via western blotting using specific antibodies (lower).
(F) CX36-deficient C20 cells generated through CRISPR/Cas9 system are validated by western blotting.
(G) Schematic diagram of experimental design. WT or CX36-deficient C20 cells (white) were co-cultured with increasing ratios of STING-deficient C20 cells (ratios ranging from 1:0.5 to 1:0.125) containing either SOD1-G85R mutants or HT-DNA (0.1 μg/mL), followed by transfection with p125-/NF-κB-pLuc. Luciferase activity was measured after 24 h.
(H) STING-deficient C20 cells expressing SOD1-G85R/93A mutants [marked with asterisk (∗)] were co-cultured with CX36-deficient cells alone or reconstituted with CX36 WT or W277A mutant plasmids. Relative expression (RE) of Ifnb1 mRNA from each designated group (i, ii, or iii) was measured with RT-qPCR.
(I) STING-deficient C20 cells expressing EV, SOD1-G85R/93A mutants [marked with asterisk (∗)] were co-cultured with either WT or CX36-deficient C20 cells. After 36 h, relative expression of indicated mRNA from designated group (i or ii) were measured via RT-qPCR. All RT-qPCR of RNA samples were normalized to β-actin. Data represent the mean of at least three independent experiments (mean ± SEM). ∗p< 0.05, ∗∗p< 0.01, ∗∗∗p< 0.001, ∗∗∗∗p< 0.0001; (C and D: unpaired Student’s t test). See also Figure S7.