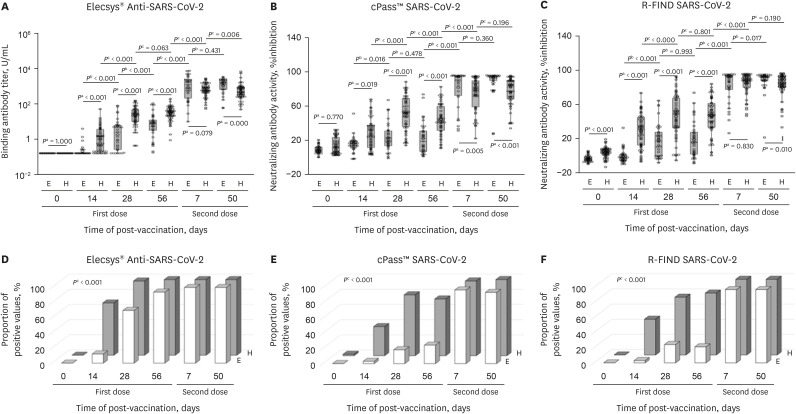
Fig. 1. Comparison of immunogenicity of COVID-19 vaccination between healthy people and patients with ESRD on hemodialysis.
(A) Binding antibody titer measured by Elecsys® Anti-SARS-CoV-2 S. (B) Neutralizing antibody activity measured by cPass™ SARS-CoV-2 Neutralization Antibody Detection Kit. (C) Neutralizing antibody activity measured by R-FIND SARS-CoV-2 Neutralizing Antibody ELISA. (D) Proportion of participants with a positive result of binding antibody measured by Elecsys® Anti-SARS-CoV-2 S. (E) Proportion of participants with a positive result of neutralizing antibody measured by cPass™ SARS-CoV-2 Neutralization Antibody Detection Kit. (F) Proportion of participants with a positive result of neutralizing antibody measured by R-FIND SARS-CoV-2 Neutralizing Antibody ELISA.
COVID-19 = coronavirus disease 2019, ESRD = end-stage renal disease, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, ELISA = enzyme-linked immunosorbent assay, E = patients with ESRD, H = healthy people.
aP values are the result of Mann–Whitney U test; bP values are the result of Wilcoxon signed-rank test; and cP values are the result of the Cochrane–Armitage test for trend.