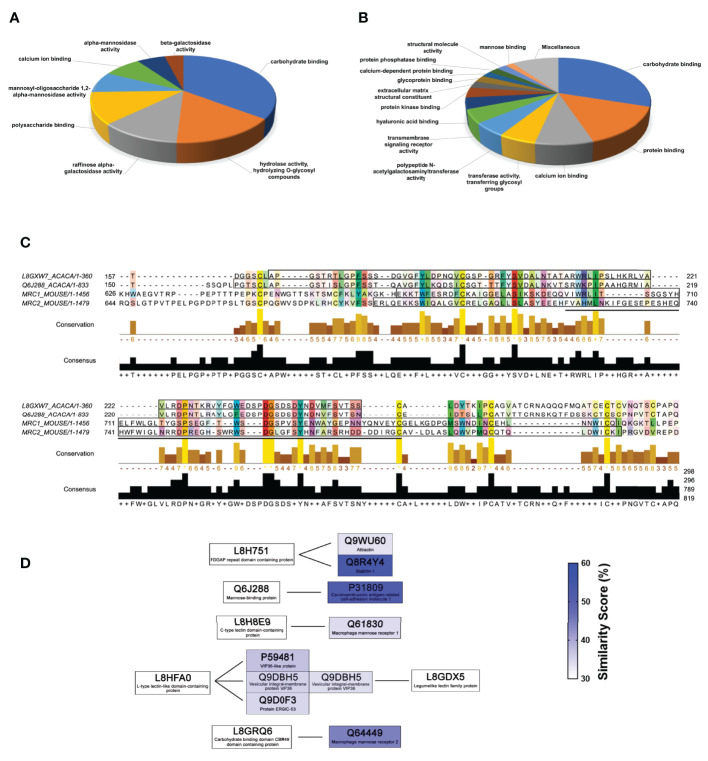
Figure 11.
Mannose-binding proteins from Acanthamoeba castellanii and RAW macrophages exhibited identity in domains with lectin properties. Proteins identified in MPPs of (A) A. castellanii (Ac) and (B) RAW 246.7 macrophages were annotated using the DAVID annotation tools and classified according to their molecular functions. For both MPPs pools, carbohydrate binding proteins comprised the main category, (C) In silico alignment of the Ac transmembrane proteins MBP (L8GXW7) and MBP1 (Q6J288) with the RAW receptors Mrc1 (Q61830) and Mrc2 (Q64449), using the multiple sequence alignment algorithm, Clustal Omega by Jalview Software. Amino acids on colored background show highly conserved residues. The DUF4114 domains of Ac proteins and C-type lectin-like domains of RAW receptors are highlighted by a black rectangle, while the domains equivalent to the secondary structure of ConA-like lectins/glucanases superfamily are indicated by black underlining. (D) From the MPPs identified in Ac, six displayed high similarity to proteins also found in the macrophage MBPs pools, suggesting molecular mimicry.