Figure 3. Ubiquitination activity of Ub‐R0RBR chimera.
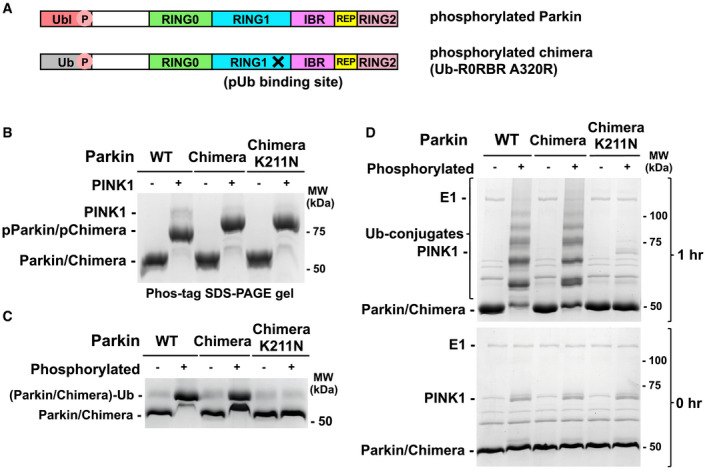
- Schematic representations of phosphorylated parkin (pParkin) and the phosphorylated chimera (pUb‐R0RBR) with the A320R mutation.
- Phosphorylation of wild‐type (WT) parkin and chimeric Ub‐R0RBR by PINK1. Reactions were analyzed on a Phos‐tag SDS–PAGE gel to assess the level of phosphorylation.
- Release of RING2 by Ub‐R0RBR chimera. Ubiquitin vinyl sulfone assays (10 μM UbVS) were performed with 3 μM phosphorylated and non‐phosphorylated full‐length parkin and Ub‐R0RBR.
- Ubiquitination activity of Ub‐R0RBR chimera. Autoubiquitination assays of phosphorylated full‐length parkin and Ub‐R0RBR (3.3 µM with 3 µm UbcH7 and 75 µM S65A ubiquitin) were performed to assess the impact of pUbl substitution by pUb on parkin activity.
