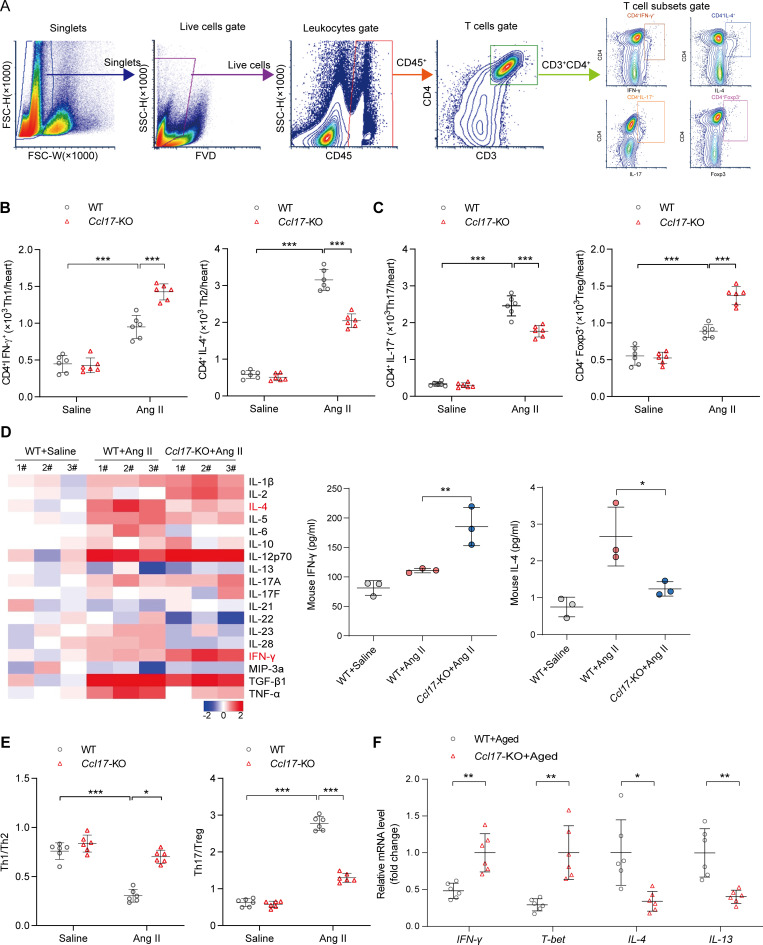
Figure 4.
CCL17 was involved in regulating T cell balance in Ang II–induced pathological cardiac hypertrophy and HF. (A) Flow cytometry gating strategy to identify T cell distribution within the cardiac tissue. Cardiac mononuclear cells were initially gated on singlets, and live cells were selected. Thereafter, live cells were characterized by the expression of the CD45+ marker (leukocytes). Finally, among the leukocytes, CD3+CD4+ T cells were further analyzed for IL-4, IFN-γ, Foxp3, or IL-17 expression. (B and C) Flow cytometry analysis of T cell subsets in 8–12-wk-old C57BL/6J background WT and Ccl17-KO mice treated with saline or Ang II for 4 wk in heart tissues (n = 6 biological replicates). (D) Th1/Th2/Th17 cytokines heatmap showed dynamic changes in 8–12-wk-old C57BL/6J background WT and Ccl17-KO mice treated with saline or Ang II for 4 wk in heart tissues (left). Quantitative results of IFN-γ and IL-4 expression levels in heart tissues are shown (right; n = 3 biological replicates). (E) Th1/Th2 and Th17/Treg ratios from 8–12-wk-old C57BL/6J background WT and Ccl17-KO mice following saline or Ang II infusion for 4 wk (n = 6 biological replicates). (F) Relative mRNA levels of Th1/2 cytokine in C57BL/6J background WT and Ccl17-KO aged mice (21 mo old) heart tissues (n = 6 biological replicates). All data are presented as the mean ± SD. The Kolmogorov–Smirnov normality test was used to assess data distribution. Statistical significance was tested using one-way ANOVA, followed by the Bonferroni post hoc test (B–E). The unpaired Student’s t-test was used for equal variance and Welch t-test for unequal variance (F). *, P < 0.05; **, P < 0.01; ***, P < 0.001.