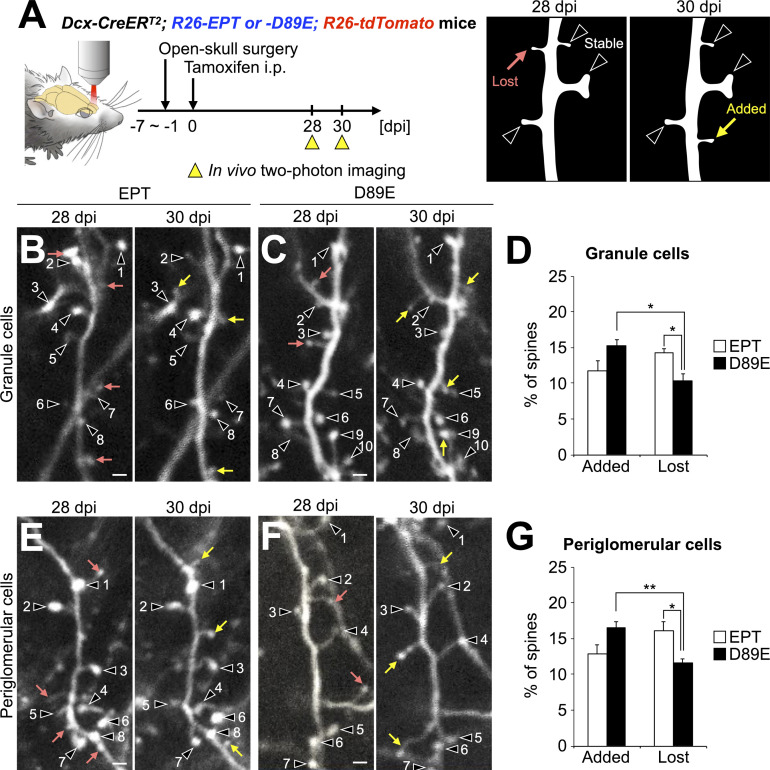
Figure 6.
PS is involved in the spine pruning of adult-born neurons in the OB. (A) Experimental scheme. In vivo two-photon imaging of dendritic spines of tdTomato+ granule and periglomerular cells in the adult Dcx-CreERT2; R26-EPT; R26-tdTomato (EPT) and Dcx-CreERT2; R26-D89E; R26-tdTomato (D89E) mice was performed at 28 and 30 dpi. Dendritic spines identified at 28 and 30 dpi were classified into stable (black arrowheads), lost (pink arrow), or added (yellow arrow; B, C, E, and F). (B–G) Effect of MFG-E8D89E on dendritic spine dynamics in tdTomato+ granule (B–D) and periglomerular (E–G) cells. Representative in vivo projection images of dendritic spines of granule (B and C) and periglomerular (E and F) cells in adult EPT (B, E; n = 4 mice) and D89E (C, F; n = 4 mice) mice are shown. Numbers identify spines that were maintained over the 2-d observation period. Percentages of added and lost spines of granule (D) and periglomerular (G) cells in the adult EPT (n = 4 mice) and D89E (n = 4 mice) mice are shown (Granule cells [D], Fgroup(1,6) = 378.7, pgroup = 1.2 × 10−6, Fdynamics(1,6) = 2.9, pdynamics = 0.90, Fgroup×dynamics(1,6) = 27.9, P = 1.9 × 10−3, two-way repeated measures ANOVA; EPT versus D89E, t(6) = 3.2, P = 0.019 in Lost, unpaired t test; added versus lost, t(3) = 5.5, P = 0.012 in D89E, paired t test; Periglomerular cells [G], Fgroup(1,6) = 332.8, pgroup = 1.7 × 10−6, Fdynamics(1,6) = 1.5, pdynamics = 0.89, Fgroup×dynamics(1,6) = 43.2, P = 5.9 × 10−4, two-way repeated measures ANOVA; EPT versus D89E, t(6) = 3.1, P = 0.021 in Lost spines, unpaired t test; added versus lost, t(3) = 7.4, P = 0.0050 in D89E, paired t test). Data are pooled from three independent experiments. Scale bars in B, C, E, F, 2 µm. *, P < 0.05; **, P < 0.01; adjusted with Bonferroni correction. Data shown are mean ± SEM.