Abstract
Background
Osimertinib—the third-generation epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI)—has been widely used as a first-line treatment for patients with metastatic EGFR-mutant non-small cell lung cancer (NSCLC). Osimertinib demonstrated central nervous system activity in patients with brain metastasis; however, its efficacy against other distant metastatic organs, including bone and liver, remains unclear. Therefore, we retrospectively analyzed the clinical efficacy of osimertinib in these patients in comparison to other EGFR-TKIs.
Methods
Clinical data of patients with advanced NSCLC receiving gefitinib/erlotinib (n = 183), afatinib (n = 55), or osimertinib (n = 150) at five medical institutions were retrospectively assessed for progression-free survival (PFS), overall survival (OS), and best overall response rate (ORR).
Results
In univariate and multivariate analyses, most distant metastases, including the brain and bone, were unrelated to the therapeutic efficacy of osimertinib, although liver metastasis and L858R mutation were independently associated with shorter PFS. PFS and OS in patients with liver metastases were significantly shorter than those in patients without liver metastases (PFS: 7.4 vs. 19.7 months, OS: 12.1 months vs. not reached, respectively). Osimertinib provided significantly longer PFS in patients with brain or bone metastasis and exon 19 deletion than the other EGFR-TKIs. The PFS of patients with liver metastases was not significantly different among the three EGFR-TKI groups. Furthermore, the ORR of osimertinib in patients with liver metastases was significantly attenuated, and the effectiveness was similar to 1st- or 2nd -generation EGFR-TKIs.
Conclusion
Osimertinib provided better clinical benefits than 1st- and 2nd-generation EGFR-TKIs for patients with EGFR-mutant NSCLC, particularly those with brain or bone metastases and exon 19 deletion; however, its efficacy against liver metastasis was remarkably attenuated. New therapeutic developments for patients with EGFR-mutant NSCLC with liver metastases are needed.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-022-09741-8.
Keywords: Distant metastases, EGFR-TKIs, EGFR mutation, Non-small cell lung cancer, Osimertinib
Background
Lung cancer is the leading cause of cancer-related mortality worldwide in 2020, and non-small cell lung cancer (NSCLC) is the most common form, accounting for 80% to 85% of all lung cancer diagnoses [1, 2]. The majority of patients with NSCLC are initially diagnosed at an advanced stage with distant metastasis, and the most frequent metastatic sites are the nervous system (20–40%), bone (20–40%), lung (15–25%), and liver (5–20%) [3–5]. Generally, patients with advanced NSCLC with brain, bone, or liver metastases are known to have worse clinical outcomes than patients without metastatic sites [3, 4].
Epidermal growth factor receptor (EGFR) mutations, which are one of the targetable driver mutations in NSCLC, are detected in approximately 50% of Asian patients and in approximately 10% of Western patients [6]. In recent decades, EGFR-tyrosine kinase inhibitors (TKIs) have significantly improved the clinical outcomes of patients with EGFR-mutant NSCLC, especially in patients with sensitizing EGFR mutations, such as exon 19 deletion and L858R point mutation [7, 8]. These common mutations account for approximately 90% of the total EGFR gene alterations [6]. Currently, multiple EGFR-TKIs, including gefitinib, erlotinib, afatinib, dacomitinib, and osimertinib, are established as standard initial treatments for patients with common EGFR mutations [7–11]. Among them, the clinical efficacies of 1st-generation EGFR-TKIs such as gefitinib and erlotinib have been adequately investigated in patients with distant metastatic sites. Several studies reported that the efficacies for patients with EGFR-mutant NSCLC with brain, bone, or liver metastases were limited, and both progression-free survival (PFS) and overall survival (OS) of these patients were remarkably shorter than those of patients without these metastatic sites [12, 13]. The median PFS and OS of patients with brain or bone metastatic sites are around 8.0–9.0 months and 20.0–25.0 months respectively, and the median PFS and OS of patients with liver metastasis are around 6.7 months and 9.2–13.4 months, respectively [12, 13], although the median PFS and OS of patients without these metastatic sites are around 11.0–15.0 months and 17.5–38.0 months, respectively. Additionally, the clinical efficacy of afatinib in patients with brain metastasis is reported to be shorter than that in patients without brain metastasis (10.1 vs 13.9 months) [14]. These results indicate that metastatic sites of EGFR-mutant NSCLC are critical factors for EGFR-TKI efficacy and directly affect patient outcomes.
The 3rd-generation irreversible EGFR-TKI, Osimertinib, can selectively inhibit both EGFR-TKI sensitizing and T790M resistance mutations, with lower activity against wild-type EGFR [15, 16]. In the double-blind phase 3 trial, FLAURA, osimertinib demonstrated significantly longer PFS and OS than the comparator regimens of gefitinib or erlotinib (median PFS: 18.9 vs. 10.2 months; HR, 0.46; P < 0.0001; median OS: 38.6 vs. 31.8 months; HR, 0.80; P = 0.0460) [17, 18], resulting that osimertinib has become a leading treatment for patients with EGFR-mutant NSCLC [17]. In the subgroup analysis of FLAURA, osimertinib demonstrated longer PFS and OS for patients with central nervous system metastases than the regimen of gefitinib or erlotinib (median PFS: 15.2 vs. 9.6 months; HR, 0.47; P < 0.0001, median OS: HR, 0.83) [17, 18]. However, the clinical efficacy of osimertinib in patients with NSCLC with other metastatic organs such as bone or liver remains unclear; although, patients with EGFR-mutant NSCLC have a higher frequency of bone metastasis than patients with EGFR-wild type [5, 19].
To investigate the clinical efficacy of osimertinib in NSCLC with various metastatic organs, we retrospectively analyzed the clinical data of 1st line treatment with osimertinib in patients with common EGFR-mutant NSCLC collected from multiple institutions. Furthermore, the efficacies of osimertinib were evaluated in comparison with those of other EGFR-TKIs in a real-world setting.
Materials and methods
Study design
This retrospective cohort study was conducted with the approval of the ethical review committee of Nagoya University Hospital (approval number:2018–017) and in accordance with the guidelines of the Declaration of Helsinki [20, 21]. We retrospectively reviewed the medical records of patients from five facilities, including Nagoya University Hospital, Konan Kosei Hospital, Kariya Toyota General Hospital, Tosei General Hospital, and Japanese Red Cross Aichi Medical Center Nagoya Daiichi Hospital. Patients enrolled in this study were selected based on the following eligibility criteria: (1) diagnosed with stage III/IV or recurrent non-squamous NSCLC, as confirmed by histological or cytological examination from January 2015 to December 2020; (2) presented with a positive EGFR mutation (exon 19 deletion or L858R point mutation); (3) were receiving 1st-generation EGFR-TKI (gefitinib or erlotinib), or 2nd-generation EGFR-TKI (afatinib), or 3rd-generation EGFR-TKI (osimertinib) for 1st line therapy. We excluded patients with no available data and non-target regions, and the time of data cut-off was August 2021. The clinical information of eligible patients, including age, sex, smoking history, histological subtype, clinical stage, performance status, treatment outcome, metastatic site, and EGFR mutation status, were retrospectively obtained from medical records. Clinical stages were assigned according to the eighth edition of the American Joint Committee on Cancer. Objective tumor responses were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [22].
EGFR mutation analysis
Genomic DNA was extracted from formalin-fixed paraffin-embedded samples with the QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany) according to manufacturer’s instructions. Target sequences in exons 19 and 21 were amplified by polymerase chain reaction and the polymerase chain reaction products were then subjected to analysis for EGFR mutations by direct Sanger sequencing.
Statistical analysis
PFS and OS were estimated using the Kaplan–Meier method and were defined as the time from the start of TKI therapy to disease progression or death, whichever was earlier, and data were censored at the last follow-up date. Gehan–Breslow–Wilcoxon and log-rank tests were implemented to analyze the differences in PFS between the patient groups. A Cox regression model was used to estimate hazard ratio (HR) and 95% confidence interval (CI). Categorical data were compared using Fisher’s exact test or the chi-square test. Statistical analyses were performed using JMP software (Version 15) and IBM SPSS Statistics (Version 28), and the differences and correlations were considered statistically significant at P < 0.05.
Results
Patient’s flowchart and characteristics
We retrospectively reviewed the clinical data of 388 eligible patients with advanced non-squamous NSCLC harboring EGFR mutations treated with EGFR-TKIs as 1st-line therapy, which were collected from five medical institutions. The flowchart of patient selection from our medical records is shown in Supplementary Fig. 1, and the enrolled patient characteristics are summarized in Table 1. The median age was 72.0 years (range 26–92), 61.6% were female, and 62.1% did not have a history of smoking. In the majority of patients, the Eastern Cooperative Oncology Group (ECOG) performance status (PS) was 0 (n = 230, 59.3%), and the clinical stage was IV (n = 270, 69.6%). Exon 19 deletions were observed in 188 patients (48.6%), and L858R point mutations were observed in 199 patients (51.4%). The number of patients with other metastatic organs included 92 (23.7%) for the contralateral lung, 160 (41.2%) for the bone, 118 (30.4%) for the brain, 34 (8.8%) for the liver, and 26 (6.7%) for the adrenal, which are consistent with the frequencies of EGFR-mutant NSCLC metastases in previous reports [3–5]. In this cohort, 183, 55, and 150 patients were treated with gefitinib, erlotinib, afatinib, and osimertinib, respectively. There were no statistically significant differences in sex, smoking status, performance status (PS), stage, and metastatic organs between gefitinib/erlotinib, afatinib, and osimertinib groups. The proportion of exon 19 deletion mutations in the afatinib group was higher than that in the other TKI groups (P < 0.0001), and the patients treated with afatinib were significantly younger than those in the other groups (P < 0.0001).
Table 1.
Clinical characteristics of 388 patients with NSCLC
| EGFR-TKIs n (%) | |||||
|---|---|---|---|---|---|
| Characteristic | Total |
Gefitinib/ Erlotinib |
Afatinib | Osimertinib | P‡ |
| 388 | 183 | 55 | 150 | ||
| Median Age (Range) | 73 (26–87) | 65 (32–79) | 72 (44–92) | < 0.0001 | |
| Gender | |||||
| Male | 149 | 64 (35.0) | 29 (52.7) | 56 (37.3) | 0.0563 |
| Female | 239 | 119 (65.0) | 26 (47.3) | 94 (62.7) | |
| Smoking statusa | |||||
| Never | 236 | 117 (64.6) | 26 (49.0) | 93 (63.7) | 0.0868 |
| Former | 104 | 49 (27.1) | 16 (30.2) | 39 (26.7) | |
| Current | 40 | 15 (8.3) | 11 (20.8) | 14 (9.6) | |
| PS | |||||
| 0 | 230 | 110 (60.1) | 33 (60.0) | 87 (58.0) | 0.7914 |
| 1 | 99 | 42 (23.0) | 15 (27.3) | 42 (28.0) | |
| ≧2 | 59 | 31 (16.9) | 7 (12.7) | 21 (14.0) | |
| Stage | |||||
| III | 15 | 9 (4.9) | 0 (0.0) | 6 (4.0) | 0.8257 |
| IV | 270 | 125 (68.3) | 40 (72.7) | 105 (70.0) | |
| Recurrence | 103 | 49 (26.8) | 15 (27.3) | 39 (26.0) | |
| Mutation statusb | |||||
| Exon 19 deletion | 188 | 76 (41.8) | 44 (80.0) | 68 (45.3) | < 0.0001 |
| L858R | 199 | 106 (58.2) | 11 (20.0) | 82 (54.7) | |
| Metastasis | |||||
| Pleura | 144 | 72 (39.3) | 16 (29.1) | 56 (37.3) | 0.3848 |
| Contralateral lung | 92 | 40 (21.9) | 12 (21.8) | 40 (26.7) | 0.5541 |
| Bone | 160 | 68 (37.2) | 29 (52.7) | 63 (42.0) | 0.1171 |
| Brain | 118 | 50 (27.3) | 20 (36.4) | 48 (32.0) | 0.3820 |
| Liver | 34 | 14 (7.7) | 3 (5.5) | 17 (11.3) | 0.3205 |
| Adrenal | 26 | 13 (7.1) | 6 (10.9) | 7 (4.7) | 0.2728 |
PS performance status
‡P values were calculated by t-test, Fisher’s exact test, or Chi-square test
aInformation was not available for 8 cases
bInformation was not available whether it was exon 19del or L858R for 1 case
Liver metastasis—an independent prognostic factor
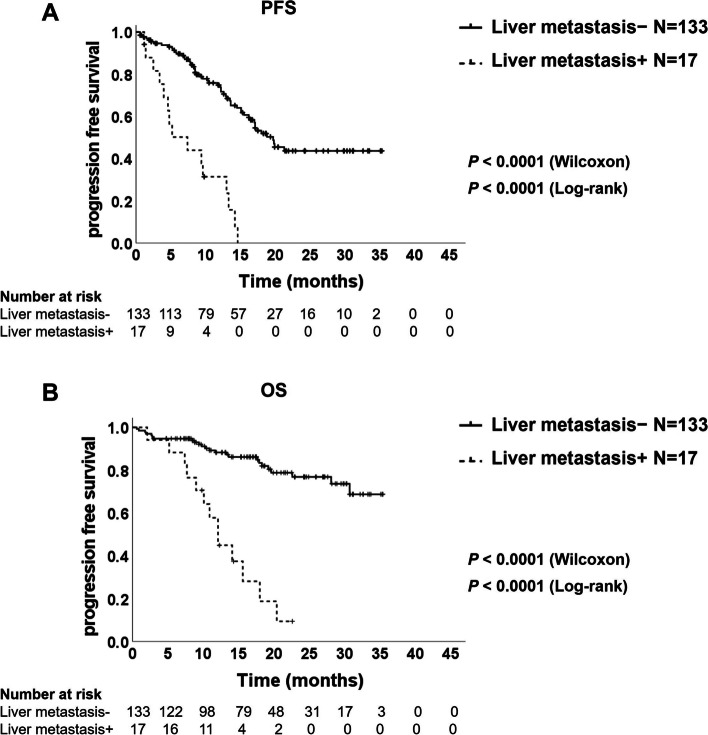
First, we performed univariate and multivariate analyses of PFS in each of the three groups. In addition to brain, bone, and liver metastases, the characteristic variables with P < 0.15 in univariate analysis of the osimertinib group were used in the multivariate analysis. In the osimertinib group, male sex, poor PS, L858R mutation, and liver metastasis were independently associated with shorter PFS (male: HR, 2.06; 95% CI, 1.25–3.38; P = 0.0045, PS1: HR, 2.70; 95% CI, 1.47–4.94; P = 0.0013, PS≧2: HR, 2.43; 95% CI, 1.16–5.11; P = 0.0193, L858R mutation: HR, 2.03; 95% CI, 1.17–3.53; P = 0.0120; liver metastasis: HR, 6.20; 95% CI, 2.87–13.38; P < 0.0001, Table 2). Among them, the PFS in patients with liver metastases was significantly shorter than that in patients without liver metastases (7.4 vs. 19.7 months; Wilcoxon P < 0.0001 and log-rank P < 0.0001; Fig. 1A), while the other distant metastatic sites were not associated with shorter PFS (Table 2). In addition, the OS in patients with liver metastases was remarkably shorter than in patients without liver metastases (12.1 months vs not reached; Wilcoxon P < 0.0001 and log-rank P < 0.0001; Fig. 1B). In the gefitinib/erlotinib group, poor PS (PS ≥ 2) was an independent poor prognostic factor (HR, 1.83; 95% CI, 1.14–2.94; P = 0.0124), although brain, bone, and liver metastases were not associated with shorter PFS (brain: HR, 1.15; 95% CI, 0.79–1.66; P = 0.4663; Bone: HR, 1.17; 95% CI, 0.83–1.66; P = 0.3712; Liver: HR, 1.44; 95% CI, 0.81–2.57; P = 0.2108, Supplementary Table 1A). Similarly, in the afatinib group, metastasis sites were not statistically associated with PFS (brain, HR, 1.42; 95% CI, 0.74–2.73; P = 0.2950; Bone, HR, 1.19; 95% CI, 0.59–2.42; P = 0.6258; Liver, HR, 2.09; 95% CI, 0.50–8.71; P = 0.3132; Supplementary Table 1B).
Table 2.
univariate and multivariate analysis of PFS in Osimertinib group
| Univariate analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% Cl | P | HR | 95% Cl | P |
| Gender | ||||||
| Female | Reference | Reference | ||||
| Male | 1.58 | 0.98–2.54 | 0.0592 | 2.06 | 1.25–3.38 | 0.0045 |
| Age | ||||||
| ≦65 years | Reference | Reference | ||||
| > 65 years | 1.58 | 0.85–2.95 | 0.1484 | 1.14 | 0.57–2.28 | 0.7027 |
| Smoking status | ||||||
| Never smoker | Reference | |||||
| Former smoker | 1.41 | 0.83–2.41 | 0.2024 | |||
| Current smoker | 1.08 | 0.46–2.57 | 0.8450 | |||
| Stage | ||||||
| Recurrence | Reference | |||||
| III | 0.82 | 0.19–3.57 | 0.7959 | |||
| IV | 1.14 | 0.66–1.97 | 0.6447 | |||
| PS | ||||||
| 0 | Reference | Reference | ||||
| 1 | 2.61 | 1.53–4.46 | 0.0004 | 2.70 | 1.47–4.94 | 0.0013 |
| ≧2 | 2.73 | 1.45–5.12 | 0.0018 | 2.43 | 1.16–5.11 | 0.0193 |
| Mutation | ||||||
| Exon 19 deletion | Reference | Reference | ||||
| L858R | 2.22 | 1.32–3.71 | 0.0025 | 2.03 | 1.17–3.53 | 0.0120 |
| Bone metastasis | ||||||
| No | Reference | Reference | ||||
| Yes | 1.19 | 0.74–1.91 | 0.4751 | 0.62 | 0.35–1.09 | 0.0956 |
| Brain metastasis | ||||||
| No | Reference | Reference | ||||
| Yes | 1.44 | 0.89–2.34 | 0.1353 | 1.27 | 0.73–2.22 | 0.3924 |
| Liver metastasis | ||||||
| No | Reference | Reference | ||||
| Yes | 5.14 | 2.81–9.41 | < 0.0001 | 6.20 | 2.87–13.38 | < 0.0001 |
| Pleura metastasis | ||||||
| No | Reference | |||||
| Yes | 1.36 | 0.84–2.19 | 0.2148 | |||
| Lung metastasis | ||||||
| No | Reference | |||||
| Yes | 1.33 | 0.79–2.23 | 0.2786 | |||
| Adrenal metastasis | ||||||
| No | Reference | Reference | ||||
| Yes | 1.98 | 0.80–4.95 | 0.1420 | 0.45 | 0.15–1.36 | 0.1581 |
PS performance status
Fig. 1.
Kaplan–Meier plot of progression-free survival (A) and overall survival (B) in the patients treated with osimertinib with and without liver metastases
Improved brain/bone metastases prognosis
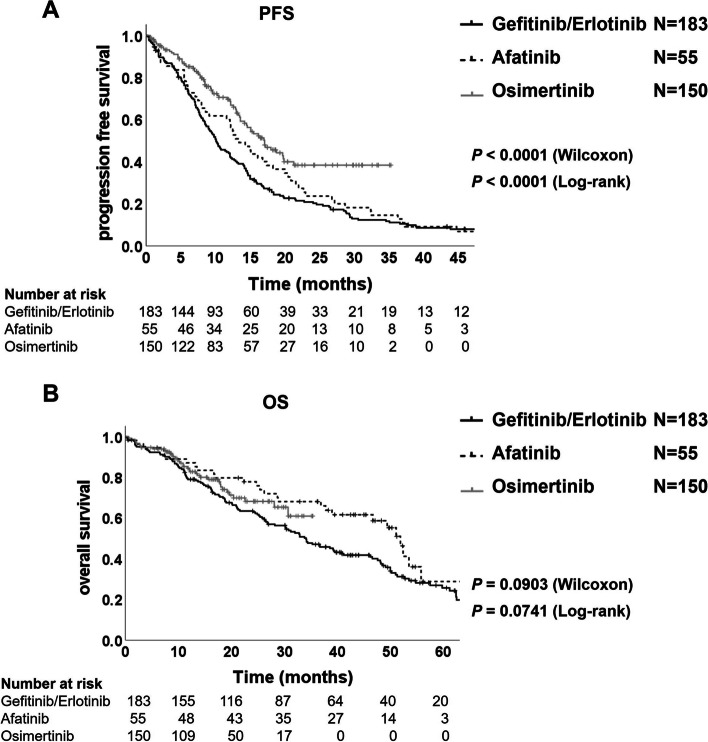
In comparison with the clinical efficacy of EGFR-TKIs, the PFS in the patients treated by osimertinib was statistically significantly longer than in the patients treated by gefitinib/erlotinib or afatinib (17.1 vs. 10.1 months; Wilcoxon P < 0.0001, log-rank P < 0.0001; HR, 0.52; 95% CI, 0.39–0.69; and 17.1 vs. 13.4 months; Wilcoxon P = 0.0541, log-rank P = 0.0250; HR, 0.65; 95% CI, 0.45–0.95; Fig. 2A). Furthermore, the patients of the osimertinib treatment group showed longer OS than the patients of the gefitinib/erlotinib treatment group, although not statistically significant (not reached vs. 34.1 months; Wilcoxon P = 0.2150 and log-rank P = 0.1818; HR, 0.77; 95% CI, 0.52–1.13; Fig. 2B), and there were no significant differences of OS in between osimertinib and afatinib groups (Fig. 2B).
Fig. 2.
Kaplan–Meier plot of progression-free survival (A) and overall survival (B) in the patients treated by gefitinib/erlotinib, afatinib or osimertinib
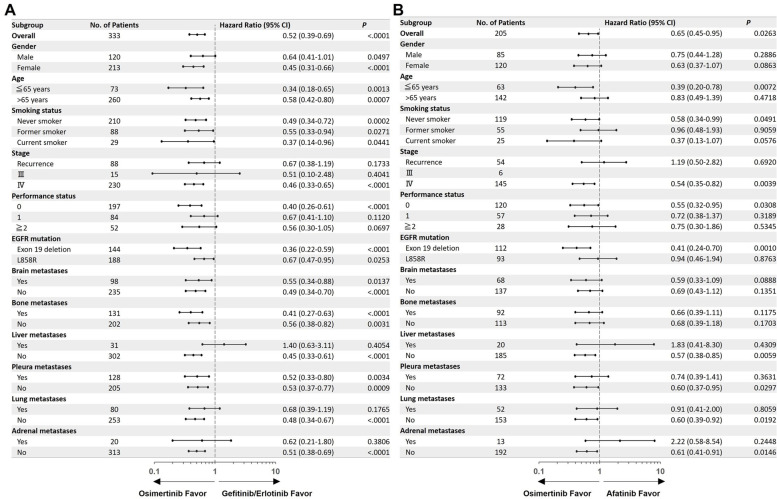
Patient characteristics with brain, bone, and liver metastases are summarized in Supplementary Table 2A, 2B, and 2C, respectively. The number of patients with brain metastases treated with stereotactic radiosurgery was significantly lower in the osimertinib group than in the other TKI groups (P = 0.0239; Supplementary Table 2A). The number of bone metastatic sites, treatment with bone-modifying agents, or radiation therapy was not significantly different between the gefitinib/erlotinib, afatinib, and osimertinib groups (Supplementary Table 2B), and the number of liver metastatic sites were also not significantly different between the gefitinib/erlotinib, afatinib, and osimertinib groups (Supplementary Table 2C). In comparison with gefitinib/erlotinib, the forest plots of PFS showed that osimertinib was associated with a significant survival benefit in brain metastases (HR, 0.55; 95% CI, 0.34–0.88; P = 0.0137), bone (HR, 0.41; 95% CI, 0.27–0.63; P < 0.0001) and pleura (HR, 0.52; 95% CI, 0.33–0.80; P = 0.0034) (Fig. 3A). On the contrary, the largest numerical differences in the hazard ratio between osimertinib and the gefitinib/erlotinib group were observed in the patients with and without liver metastases (HR, 1.40; 95% CI, 0.63–3.11; P = 0.4054); however, there were no statistical differences between osimertinib and gefitinib/erlotinib in the subgroup with contralateral lung metastases and adrenal metastases (Fig. 3A). The second largest numerical difference was observed between exon 19 deletion and L858R mutations (Fig. 3A). Similar to previous studies [17], osimertinib was associated with a significant survival benefit in the exon 19 deletion subgroup (HR, 0.36; 95% CI, 0.22–0.59; P < 0.0001) compared to the L858R subgroup (HR, 0.67; 95% CI, 0.47–0.95; P = 0.0253).
Fig. 3.
Subgroup analyses of progression-free survival. Osimertinib compared with gefitinib/erlotinib (A) or afatinib (B). A hazard ratio of less than one implies a lower risk of disease progression or death with osimertinib than with other EGFR-TKIs. Smoking status were not available for 2 patients in the gefitinib/erlotinib group, 2 patients in the afatinib group and 4 patients in the osimertinib group. Information was not available whether it was exon 19 deletion or L858R mutation for 1 patient in gefitinib/erlotinib group. There were no patient with stage III in afatinib group. CI, confidence interval
Similarly, in comparison with afatinib, osimertinib was associated with better survival benefit in the brain and bone metastases subgroup, although the difference was not statistically significant (brain: HR, 0.59; 95% CI, 0.33–1.09; P = 0.0888; Bone: HR, 0.66; 95% CI, 0.39–1.11; P = 0.1175) (Fig. 3B). In contrast, the largest numerical differences were observed between patients with and without liver or adrenal metastases, indicating that the clinical benefits of osimertinib are weak in patients with liver metastases and adrenal metastases (liver: HR, 1.83; 95% CI, 0.41–8.30; P = 0.4309, adrenal; HR, 2.22; 95% CI, 0.58–8.54; P = 0.2448; Fig. 3B). Additionally, large numerical differences were observed between the exon 19 deletion and L858R mutation, indicating that osimertinib was associated with a significant survival benefit in the exon 19 deletion subgroup (HR, 0.41; 95% CI, 0.24–0.70; P = 0.0010), but not in the L858R subgroup (HR, 0.94; 95% CI, 0.46–1.94; P = 0.8763) (Fig. 3B).
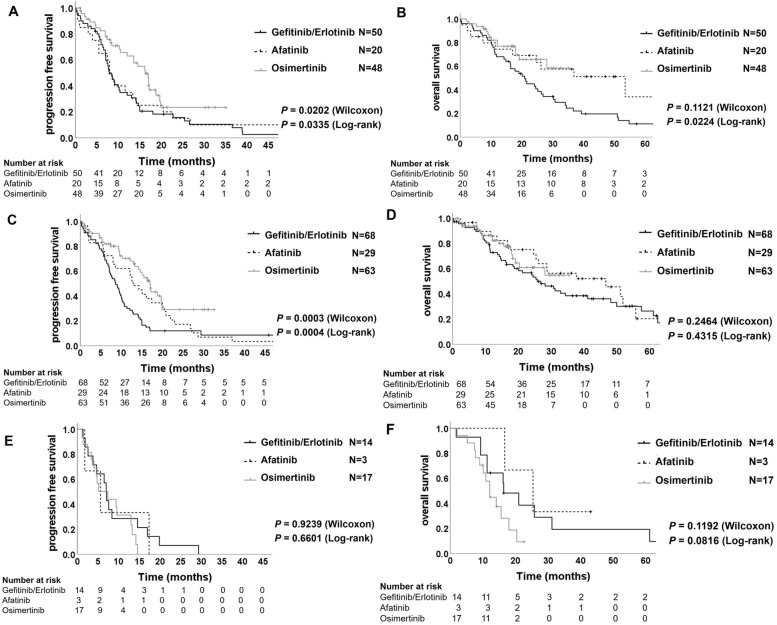
Subsequently, we analyzed the clinical efficacy of EGFR-TKIs in patients with distant organ metastasis using the Kaplan–Meier estimator. The PFS of the patients with brain metastasis treated by osimertinib was significantly longer than those of the patients of the gefitinib/erlotinib group (16.3 vs. 7.9 months; Wilcoxon P = 0.0075 and log-rank P = 0.0120), and the afatinib group (16.3 vs. 8.3 months; Wilcoxon P = 0.0347 and log-rank P = 0.0845) (Fig. 4A). Furthermore, the OS in the patients with brain metastasis treated by osimertinib was significantly longer than in the patients treated by gefitinib/erlotinib (not reached vs. 20.9 months; Wilcoxon P = 0.0725 and log-rank P = 0.0326), while there was not significantly difference between the osimertinib and the afatinib group (not reached vs. 53.5 months; Wilcoxon P = 0.6219 and log-rank P = 0.8118) (Fig. 4B). These results indicate that the clinical efficacy of osimertinib for patients with EGFR-mutant NSCLC with brain metastases is equal to or greater than that of the other EGFR-TKIs, and our analyzed data were consistent with the previous reports of the FLAURA trial [17, 18]. Furthermore, in patients with bone metastasis, the PFS of the osimertinib group was significantly longer than that of the gefitinib/erlotinib group (17.0 vs. 8.6 months; Wilcoxon P < 0.0001 and log-rank P < 0.0001; Fig. 4C) and showed a better trend compared with those of the patients in the afatinib group (17.0 vs. 12.9 months; Wilcoxon P = 0.1884 and log-rank P = 0.1144; Fig. 4C), although the OS demonstrated no significant differences among the three EGFR-TKI groups (Fig. 4D). However, the PFS in the patients with liver metastasis of osimertinib group showed no superiority to the patients of the gefitinib/erlotinib group (7.4 vs. 7.1 months; Wilcoxon P = 0.7406 and log-rank P = 0.3997; Fig. 4E) and the afatinib group (7.4 vs. 5.6 months; Wilcoxon P = 0.8674 and log-rank P = 0.4247; Fig. 4E). Similar to the PFS analyses, the OS of patients with liver metastasis treated with osimertinib showed no significant difference compared to the patients treated with 1st- or 2nd-generation EGFR-TKIs (Fig. 4F). However, the PFS in patients without liver metastasis was significantly better in the osimertinib group than in the other EGFR-TKI groups (Supplementary Fig. 2A). The OS in the patients without liver metastasis in the osimertinib group was significantly better than that of the patients in the gefitinib/erlotinib group, while there was no significant difference between the osimertinib and afatinib groups (Supplementary Fig. 2B). These results indicate that liver metastasis critically affects the clinical efficacy of osimertinib in patients with EGFR-mutant NSCLC.
Fig. 4.
Kaplan–Meier plot of progression-free survival and of overall survival in the patients with brain metastases (A) and (B), respectively, in the patients with bone metastases (C) and (D), respectively, and in the patients with liver metastases (E) and (F), respectively
Attenuated clinical efficacy of osimertinib
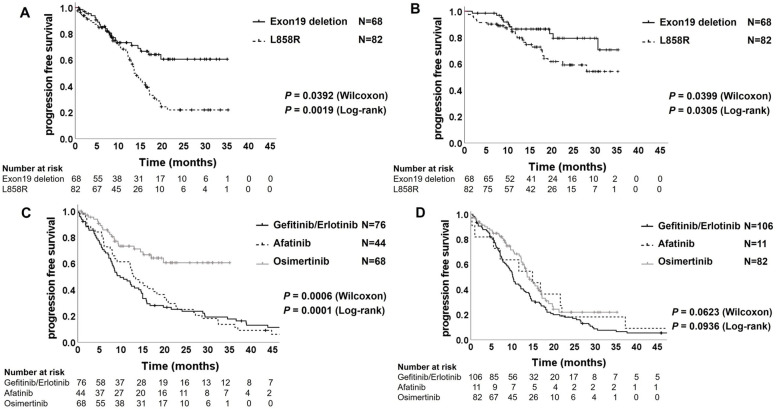
In patients treated with osimertinib, the PFS and OS of patients with exon 19 deletions were significantly longer than those of patients with the L858R mutation (Fig. 5A and Fig. 5B). As expected, the PFS in the patients with exon 19 deletion mutation in the osimertinib group was significantly better than that in the patients in the gefitinib/erlotinib group and the afatinib group (not reached vs. 9.8 months; Wilcoxon P = 0.0001 and log-rank P < 0.0001, and not reached vs. 13.2 months; Wilcoxon P = 0.0085 and log-rank P = 0.0007; Fig. 5C). However, the PFS of the patients with L858R mutation did not show a significant difference between the osimertinib group and the afatinib group (13.6 vs. 14.9 months; Wilcoxon P = 0.6038 and log-rank P = 0.8761; Fig. 5C); though, osimertinib showed superior median PFS to gefitinib/erlotinib (13.6 vs. 10.2 months; Wilcoxon P = 0.0176 and log-rank P = 0.0239; Fig. 5D). On the other hand, the OS of patients with both exon 19 deletion and L858 mutation in the osimertinib group were not significantly different from those in the other EGFR-TKI groups (Supplementary Fig. 3A and 3B).
Fig. 5.
Kaplan–Meier plot of progression-free survival (A) and overall survival (B) in the patients treated by osimertinib with exon 19 deletion or L858R mutation. Kaplan–Meier plot of progression-free survival in the patients with exon 19 deletion mutation (C) and in the patients with L858R mutation (D) treated by gefitinib/erlotinib, afatinib or osimertinib
Limited response rate of osimertinib
To assess the response rate to osimertinib, we compared the ORRs in patients treated with each EGFR-TKI. The ORRs were 68.2% (116/170) in the gefitinib/erlotinib group, 59.6% (31/52) in the afatinib group, and 73.9% (99/134) in the osimertinib group, indicating that osimertinib demonstrated a slightly better response rate than the other EGFR-TKIs (Table 3). However, the response rate in patients with liver metastasis treated with osimertinib was remarkably reduced, and the ORRs were 53.3% (8/15), while those of the gefitinib/erlotinib and afatinib groups were 57.1% (8/14) and 66.7% (2/3), respectively (Table 4 and Supplementary Fig. 4A). Consistent with the results of PFS and OS, the clinical response to osimertinib in patients with liver metastases was significantly attenuated, and the effectiveness was similar to 1st- or 2nd-generation EGFR-TKIs.
Table 3.
Best response in each treatment population
|
Gefitinib/Erlotinib n (%) |
Afatinib n (%) |
Osimertinib n (%) |
|
|---|---|---|---|
| Totala | 170 | 52 | 134 |
| Best Response | |||
| CR | 17 (10.0) | 5 (9.6) | 5 (3.7) |
| PR | 99 (58.2) | 26 (50.0) | 94 (70.1) |
| SD | 38 (22.4) | 15 (28.8) | 26 (19.4) |
| PD | 16 (9.4) | 6 (11.5) | 9 (6.7) |
| ORR | 116 (68.2) | 31 (59.6) | 99 (73.9) |
| DCR | 154 (90.6) | 46 (88.5) | 125 (93.3) |
Table 4.
Best response in patients with/without liver metastasis
| Gefitinib/Erlotinib | Afatinib | Osimertinib | ||||
|---|---|---|---|---|---|---|
| Liver metastasis + n (%) |
Liver metastasis- n (%) |
Liver metastasis + n (%) |
Liver metastasis- n (%) |
Liver metastasis + n (%) |
Liver metastasis- n (%) |
|
| Total a | 14 | 156 | 3 | 49 | 15 | 119 |
| Best Response | ||||||
| CR | 0 (0.0) | 17 (10.9) | 0 (0.0) | 5 (10.2) | 0 (0.0) | 5 (4.2) |
| PR | 8 (57.1) | 91 (58.3) | 2 (66.7) | 24 (48.9) | 8 (53.3) | 86 (72.3) |
| SD | 2 (14.3) | 36 (23.1) | 0 (0.0) | 15 (30.6) | 4 (26.7) | 22 (18.5) |
| PD | 4 (28.6) | 12 (7.7) | 1 (33.3) | 5 (10.2) | 3 (20.0) | 6 (5.0) |
| ORR | 8 (57.1) | 108 (69.2) | 2 (66.7) | 29 (59.2) | 8 (53.3) | 91 (76.5) |
| DCR | 10 (71.4) | 144 (92.3) | 2 (66.7) | 44 (89.8) | 12 (80.0) | 113 (95.0) |
In addition, in patients with exon 19 deletion mutations, the ORR of the osimertinib group was 76.2% (48/63), whereas that of the gefitinib/erlotinib and afatinib groups was 66.7% (46/69) and 57.1% (24/42), respectively (Table 5 and Supplementary Fig. 4B). However, in patients with L858R mutations, the ORRs of the osimertinib group were 71.8% (51/71), whereas those of the gefitinib/erlotinib and afatinib groups were 70.0% (70/100) and 70.0% (7/10), respectively (Table 5 and Supplementary Fig. 4B). In addition to the analyses of PFS and OS, the ORRs in patients with exon 19 deletions treated with osimertinib were superior to those in patients with other EGFR-TKIs; while those in patients with L858R did not show remarkable differences among the three groups.
Table 5.
Best response in patients by EGFR mutations
| Gefitinib/Erlotinib | Afatinib | Osimertinib | ||||
|---|---|---|---|---|---|---|
|
Exon 19 del n (%) |
L858R n (%) |
Exon 19 del n (%) |
L858R n (%) |
Exon 19 del n (%) |
L858R n (%) |
|
| Totala | 69 | 100 | 42 | 10 | 63 | 71 |
| Best Response | ||||||
| CR | 11 (15.9) | 6 (6.0) | 3 (7.1) | 2 (20.0) | 3 (4.8) | 2 (2.8) |
| PR | 35 (50.7) | 64 (64.0) | 21 (50.0) | 5 (50.0) | 45 (71.4) | 49 (69.0) |
| SD | 16 (23.2) | 22 (22.0) | 14 (33.3) | 1 (10.0) | 9 (14.3) | 17 (24.20) |
| PD | 7 (10.1) | 8 (8.0) | 4 (9.5) | 2 (20.0) | 6 (9.5) | 3 (4.2) |
| ORR | 46 (66.7) | 70 (70.0) | 24 (57.1) | 7 (70.0) | 48 (76.2) | 51 (71.8) |
| DCR | 62 (89.9) | 92 (92.0) | 38 (90.5) | 8 (80.0) | 57 (90.5) | 68 (95.8) |
CR complete response, PR partial response, SD stable disease, PD progressive disease, ORR objective response rate, DCR disease control rate, exon 19 del exon 19 deletion
aThere were 13 patients in the gefitinib/erlotinib group, 3 cases in the afatinib group, and 16 cases in the osimertinib group could not be evaluated
Discussion
To our knowledge, this is the first report of the efficacy of osimertinib in comparison with other EGFR-TKIs in a real-world setting. Overall, osimertinib provided better clinical benefits to patients with EGFR-mutated NSCLC, particularly those with brain and bone metastases, and exon 19 deletion than 1st- and 2nd-generation EGFR-TKIs, while the efficacy was attenuated in patients with liver metastases and L858R mutation.
In this study, PFS, OS, and ORRs of patients treated with osimertinib or 1st-generation EGFR-TKIs were similar to the results of the FLAURA trial, and the HR of PFS was also very close to the trial result. Furthermore, our study demonstrated good clinical efficacy of osimertinib for patients with brain metastasis and exon 19 deletion, and these results are consistent with a previous report [17, 18]. Among various distant metastatic sites, this study is the first to demonstrate the clinical efficacy of osimertinib in patients with bone metastases who had a poor prognosis in the case of 1st-generation EGFR-TKIs treatments [12]. In vivo analysis showed that osimertinib effectively regressed tumors in an EGFR-mutant bone metastatic model [23], indicating that the tissue penetration rate of osimertinib can be greater than that of other EGFR-TKIs. However, our study showed remarkably lower efficacy of osimertinib as well as other EGFR-TKIs in patients with liver metastasis. Moreover, the same trend was observed in another retrospective study [24], suggesting that the clinical efficacy of any EGFR-TKI monotherapy is limited to NSCLC with liver metastasis. In the tumor microenvironment of the liver metastatic site, the expression level of vascular endothelial growth factor (VEGF) is increased in comparison with other metastatic sites [25]. VEGF induces not only tumor angiogenesis, but also promotes the proliferation of EGFR-mutant cancer cells and affects the immune suppressive network [26–28]. In fact, combination therapy with erlotinib plus anti-VEGF receptor antibody, ramucirumab, shows better HR of PFS in patients with liver metastases than erlotinib monotherapy [29]. Furthermore, insulin-like growth factor 1 (IGF-1), which is a ligand of the IGF-1 receptor (IGF-1R), is abundantly expressed in the microenvironment of the liver metastatic site, and the signaling from IGF-1R promotes the tolerance to osimertinib in EGFR-mutant tumors [30]. These liver-specific tumor microenvironments may reduce the clinical efficacy of osimertinib in patients with liver metastasis.
Similar to previous reports [17, 18], our study also demonstrated that the clinical efficacy of osimertinib was limited in patients with the L858R mutation, although osimertinib showed longer median PFS than 1st-generation TKIs. Osimertinib was developed for its activity against the T790M mutation, which is a secondary point mutation in EGFR and is the most common resistance mechanism to 1st- and 2nd-generation EGFR-TKIs [31, 32]. A recent study showed that the prevalence of the T790M mutation was significantly higher in patients with exon 19 deletions than in those with the L858R mutation (50.4% versus 36.5%) [33], indicating that osimertinib is more beneficial in patients with exon 19 deletions. Our data showed that the PFS of patients with exon 19 deletion in the osimertinib group was significantly longer than that in the afatinib group; however, there was no significant difference in PFS in patients with the L858R mutation between osimertinib and afatinib. To investigate the clinical benefits of osimertinib compared with afatinib in patients with the L858R mutation, further studies are needed.
Although this study showed the superiority of osimertinib in PFS over afatinib, there were no remarkable differences in OS between the two EGFR-TKIs. In our cohort, the afatinib group included a high proportion of patients with exon19 deletion mutation and relatively younger patients. Furthermore, after the failure of 1st-line gefitinib/erlotinib or afatinib, osimertinib was used for 33.3% patients of the gefitinib/erlotinib group and 27.3% patients of the afatinib group as 2nd-line treatment (Supplementary Table 3), while the osimertinib group did not include other EGFR-TKIs therapy after 2nd-line treatments. Most T790M-positive patients were treated with osimertinib as 2nd-line treatment after 1st-line afatinib (Supplementary Table 3). These factors may have affected the good OS of the afatinib group. Furthermore, this study has some limitations, including retrospectively analyzed results and a limited population, especially the number of patients treated with afatinib. In addition, OS analysis required a longer follow-up period, particularly for patients treated with osimertinib. To clearly show the clinical benefits of osimertinib in comparison with 2nd-generation EGFR-TKIs, large-scale clinical trials are necessary.
Conclusion
The clinical efficacy of osimertinib was better in most cases of EGFR-mutant NSCLC than in those of 1st- or 2nd-generation EGFR-TKIs. Osimertinib provided significantly better clinical outcomes in patients with exon 19 deletions and brain or bone metastases and demonstrated significantly shorter PFS in patients with liver metastasis than in patients with other metastatic organs. The clinical efficacy did not show superiority to 1st- and 2nd-generation EGFR-TKIs. These results indicate that new therapeutic strategies for patients with EGFR-mutant NSCLC with liver metastasis are urgently needed.
Supplementary Information
Additional file 1: Supplementary Table 1A. univariate and multivariateanalysis of PFS in Gefitinib/Erlotinib group. Supplementary Table 1B. univariate and multivariate analysis of PFS in Afatinib group. Supplementary Table 2A. Clinicalcharacteristics of 118 patients with brain metastases. Supplementary Table 2B. Clinical characteristics of 160 patientswith bone metastases. Supplementary Table2C. Clinical characteristics of 34 patients with liver metastases. Supplementary Table 3. Second-linetreatments afterGefitinib/Erlotinib or Afatinib failure. Supplementary Figure 1.Flowchartof patient selection in this cohort. Supplementary Figure 2. Kaplan–Meier plot ofprogression-free survival (A) and overall survival (B) in patients withoutliver metastases. Supplementary Figure 3. Kaplan–Meier plot ofoverall survival in patients with exon 19 deletion mutation (A) in patientswith the L858R mutation (B). Supplementary Figure 4. Objective response ratein three EGFR-TKI groups with or without liver metastasis (A) and exon 19deletion or L858R (B). Tumor responses were assessed by the investigatorsaccording to the Response Evaluation Criteria in Solid Tumors (RECIST) version1.1. P values were calculated using the chi-square test. Thirteen casesin the gefitinib/erlotinib group, three in the afatinib group, and 16 in theosimertinib group could not be evaluated.
Acknowledgements
Not applicable.
Abbreviations
- EGFR
Epidermal Growth Factor Receptor
- NSCLC
Non-Small Cell Lung Cancer
- ORR
Overall Response Rate
- OS
Overall Survival
- PFS
Progression-Free Survival
- TKI
Tyrosine Kinase Inhibitor
- PS
Performance Status
Authors’ contributions
S.G. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of data analysis, including and especially any adverse effects. I.T. contributed to the conception and design of this study. S.G. completes the manuscript, and I.T. contributes to the revision of the manuscript. All authors contributed to the study, and all authors agree with the manuscript to be published.
Funding
This study did not receive any specific grant from any funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted with the approval of the ethical review committee of Nagoya University Hospital (approval number:2018–017) and in accordance with the guidelines of the Declaration of Helsinki. The study is retrospective cohort study and only anonymized clinical information of patients was collected without interfering with patients’ treatment plans or posing physiological risks to patients. Informed consent was waived by the same ethical review committee of Nagoya University Hospital. Information about this research, including the purpose, is available on the website of Nagoya University School of Medicine and patients are given the opportunity to refuse to participate in this study.
Consent for publication
Not applicable.
Competing interests
T.H. received research funding from Boehringer Ingelheim. N.H. reported receiving grant from Boehringer Ingelheim; Pfizer Inc.; Astellas Pharma Inc.; Ono Pharmaceutical Co.; Shionogi & Co.; AstraZeneca; Sanofi K.K.; Teijin Limited; MSD K.K.; Meiji Seika Pharma Co.; Daiichi Sankyo Company, Limited; GlaxoSmithKline K.K.; Otsuka Pharmaceutical Co.; KYORIN Pharmaceutical Co., Ltd.; Sumitomo Dainippon Pharma Co.; Novartis Pharma K.K.; Kyowa Hakko Kirin Co.; Eli Lilly Japan K.K.; and Chugai Pharmaceutical Co. that was paid to Nagoya University. Other authors report no conflict of interest in this work.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Cancer - Key Facts. https://www.who.int/news-room/factsheets/detail/cancer. Accessed 30th Nov 2021.
- 2.Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14(8):535–546. doi: 10.1038/nrc3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riihimäki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86(1):78–84. doi: 10.1016/j.lungcan.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 4.Hess KR, Varadhachary GR, Taylor SH, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106(7):1624–1633. doi: 10.1002/cncr.21778. [DOI] [PubMed] [Google Scholar]
- 5.Kuijpers CCHJ, Hendriks LEL, Derks JL, et al. Association of molecular status and metastatic organs at diagnosis in patients with stage IV non-squamous non-small cell lung cancer. Lung Cancer. 2018;121:76–81. doi: 10.1016/j.lungcan.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Li K, Yang M, Liang N, et al. Determining EGFR-TKI sensitivity of G719X and other uncommon EGFR mutations in non-small cell lung cancer: Perplexity and solution (Review) Oncol Rep. 2017;37(3):1347–1358. doi: 10.3892/or.2017.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28 Supp 1(Suppl 1):S24–31. doi: 10.1038/onc.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer. 2010;10(11):760–774. doi: 10.1038/nrc2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park K, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17(5):577–589. doi: 10.1016/S1470-2045(16)30033-X. [DOI] [PubMed] [Google Scholar]
- 10.Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(11):1454–1466. doi: 10.1016/S1470-2045(17)30608-3. [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network: NCCN flash updates: NCCN clinical practice guidelines and NCCN compendium updated for Non-Small Cell Lung Cancer (NSCLC) version 2. 2020. Published 2020. Accessed 26th Mar 2021.
- 12.Yoshihiko T, Akihiro T, Kenji N, et al. Impact of metastatic status on the prognosis of EGFR mutation-positive non-small cell lung cancer patients treated with first-generation EGFR-tyrosine kinase inhibitors. Oncol Lett. 2017;14(6):7589–7596. doi: 10.3892/ol.2017.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuan-Li Wu, Tsai M-J, Yang C-J, et al. Liver metastasis predicts poorer prognosis in stage IV lungadenocarcinoma patients receiving first-line gefitinib. Lung Cancer. 2015;88(2):187–194. doi: 10.1016/j.lungcan.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 14.de Marinis F, Laktionov KK, Poltoratskiy A, et al. Afatinib in EGFR TKI-naïve patients with locally advanced or metastatic EGFR mutation-positive non-small cell lung cancer: Interim analysis of a Phase 3b study. Lung Cancer. 2021;152:127–134. doi: 10.1016/j.lungcan.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Cross DA, Ashton SE, Ghiorghiu S, et al. an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4(9):1046–1061. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pao W, Chmielecki J. Rational, CNS response to osimertinib in patients (pts) with T790M-positive advanced NSCLC: data from a randomized phase III trial (AURA3) J Clin Oncol. 2017;35(15):Suppl:9005. [Google Scholar]
- 17.Soria J-C, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 18.Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 19.Doebele RC, Lu X, Sumey C, et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer. 2012;118(18):4502–4511. doi: 10.1002/cncr.27409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka I, Morise M, Kodama Y, et al. Potential for afatinib as an optimal treatment for advanced non-small cell lung carcinoma in patients with uncommon EGFR mutations. Lung Cancer. 2019;127:169–171. doi: 10.1016/j.lungcan.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka I, Morise M, Miyazawa A, et al. Potential benefits of bevacizumab combined with platinum-based chemotherapy in advanced non-small-cell lung cancer patients with EGFR mutation. Clin Lung Cancer. 2020;21(3):273–280. doi: 10.1016/j.cllc.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Higuchi T, Sugisawa N, Park JH, et al. Osimertinib regressed an EGFR-mutant lung-adenocarcinoma bone-metastasis mouse model and increased long-term survival. Transl Oncol. 2020;13(10):100826. doi: 10.1016/j.tranon.2020.100826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakata Y, Sakata S, Oya Y, et al. Osimertinib as first-line treatment for advanced epidermal growth factor receptor mutation–positive non–small-cell lung cancer in a real-world setting (OSI-FACT) Eur J Cancer. 2021;159:144–153. doi: 10.1016/j.ejca.2021.09.041. [DOI] [PubMed] [Google Scholar]
- 25.Chen DS, Hurwitz H, et al. Combinations of Bevacizumab With Cancer Immunotherapy. Cancer J. 2018;24(4):193–204. doi: 10.1097/PPO.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 26.Goel HL, Mercurio AM. VEGF targets the tumour cell. In Nat Rev Cancer. 2013;13(12):871–882. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le X, Nilsson M, Goldman J, et al. Dual EGFR-VEGF Pathway Inhibition: A promising strategy for patients with EGFR-mutant NSCLC. J Thorac Oncol. 2021;16(2):205–215. doi: 10.1016/j.jtho.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Fukumura D, Kloepper J, Amoozgar Z, et al. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325–340. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(12):1655–1669. doi: 10.1016/S1470-2045(19)30634-5. [DOI] [PubMed] [Google Scholar]
- 30.Taniguchi H, Yamada T, Wang R, et al. AXL confers intrinsic resistance to osimertinib and advances the emergence of tolerant cells. Nat Commun. 2019;10(1):259. doi: 10.1038/s41467-018-08074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minari R, Bordi P, Tiseo M. Third-generation epidermal growth factor receptor-tyrosine kinase inhibitors in T790M-positive non-small cell lung cancer: Review on emerged mechanisms of resistance. Transl Lung Cancer Res. 2016;5(6):695–708. doi: 10.21037/tlcr.2016.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arcila ME, Oxnard GR, Nafa, et al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res. 2011;17(5):1169–1180. doi: 10.1158/1078-0432.CCR-10-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ke EE, Zhou Q, Zhang QY, et al. A Higher Proportion of the EGFR T790M Mutation May Contribute to the Better Survival of Patients with Exon 19 Deletions Compared with Those with L858R. J Thorac Oncol. 2017;12(9):1368–1375. doi: 10.1016/j.jtho.2017.05.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1A. univariate and multivariateanalysis of PFS in Gefitinib/Erlotinib group. Supplementary Table 1B. univariate and multivariate analysis of PFS in Afatinib group. Supplementary Table 2A. Clinicalcharacteristics of 118 patients with brain metastases. Supplementary Table 2B. Clinical characteristics of 160 patientswith bone metastases. Supplementary Table2C. Clinical characteristics of 34 patients with liver metastases. Supplementary Table 3. Second-linetreatments afterGefitinib/Erlotinib or Afatinib failure. Supplementary Figure 1.Flowchartof patient selection in this cohort. Supplementary Figure 2. Kaplan–Meier plot ofprogression-free survival (A) and overall survival (B) in patients withoutliver metastases. Supplementary Figure 3. Kaplan–Meier plot ofoverall survival in patients with exon 19 deletion mutation (A) in patientswith the L858R mutation (B). Supplementary Figure 4. Objective response ratein three EGFR-TKI groups with or without liver metastasis (A) and exon 19deletion or L858R (B). Tumor responses were assessed by the investigatorsaccording to the Response Evaluation Criteria in Solid Tumors (RECIST) version1.1. P values were calculated using the chi-square test. Thirteen casesin the gefitinib/erlotinib group, three in the afatinib group, and 16 in theosimertinib group could not be evaluated.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.