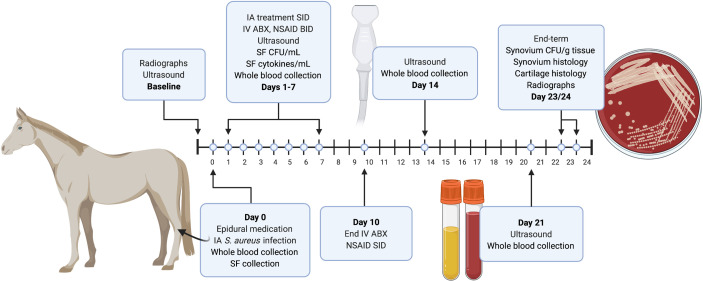
Figure 1.
Diagram of the experimental design and timing of major events such as sample collection and ultrasound. Baseline diagnostic imaging and epidural catheter placement was performed 24-48 hours prior to the start of the study. Baseline radiographs and ultrasound images were taken 3-5 days before the start of the study (day 0). On day 0, samples (blood and synovial fluid) were collected, epidural medication was administered, and S. aureus was injected intra-articularly (IA) into one randomly chosen tarsocrural joint. Beginning 24 hours post-infection (day 1), horses were treated with 500mg amikacin alone (control AMK; n=6) or BIO-PLY and 500mg of amikacin (AMK+BIO-PLY treatment; n=6) daily for 7 days. Blood and synovial fluid (SF) samples were collected at baseline (day 0) and from days 1-7. Synovial fluid was processed to determine bacterial load (CFU/mL) and cytokine milieu. Whole blood was submitted for in-house complete blood counts and plasma was saved for systemic biomarker analysis. All horses received intravenous (IV) antimicrobials (ABX) and analgesics (NSAID) twice per day (BID) for 7-10 days. From day 7 to 21, NSAIDs were administered once per day (SID). Ultrasound exams were performed at day 14 and 21. At end-term (day 23-24), horses had post-infection radiographs performed, were euthanized, and had samples (synovium and cartilage) collected at autopsy for microbiology and histopathology. Synovium was processed for bacterial load (CFU/g). Synovium and cartilage were harvested and submitted for histopathology.