Abstract
Introduction
Sepsis is a common, potentially life-threatening complication of infection. The optimal treatment for sepsis includes prompt antibiotics and intravenous fluids, facilitated by its early and accurate recognition. Currently, clinicians identify and assess severity of suspected sepsis using validated clinical scoring systems. In England, the National Early Warning Score 2 (NEWS2) has been mandated across all National Health Service (NHS) trusts and ambulance organisations. Like many clinical scoring systems, NEWS2 should not be used without clinical judgement to determine either the level of acuity or a diagnosis. Despite this, there is a tendency to overemphasise the score in isolation in patients with suspected infection, leading to the overprescription of antibiotics and potentially treatment-related complications and rising antimicrobial resistance. The biomarker procalcitonin (PCT) has been shown to be useful in specific circumstances to support appropriate antibiotics prescribing by identifying bacterial infection. PCT is not routinely used in the care of undifferentiated patients presenting to emergency departments (EDs), and the evidence base of its optimal usage is poor. The PROcalcitonin and NEWS2 evaluation for Timely identification of sepsis and Optimal (PRONTO) study is a randomised controlled trial (RCT) in adults with suspected sepsis presenting to the ED to compare standard clinical management based on NEWS2 scoring plus PCT-guided risk assessment with standard clinical management based on NEWS2 scoring alone and compare if this approach reduces prescriptions of antibiotics without increasing mortality.
Methods and analysis
PRONTO is a parallel two-arm open-label individually RCT set in up to 20 NHS EDs in the UK with a target sample size of 7676 participants. Participants will be randomised in a ratio of 1:1 to standard clinical management based on NEWS2 scoring or standard clinical management based on NEWS2 scoring plus PCT-guided risk assessment. We will compare whether the addition of PCT measurement to NEWS2 scoring can lead to a reduction in intravenous antibiotic initiation in ED patients managed as suspected sepsis, with at least no increase in 28-day mortality compared with NEWS2 scoring alone (in conjunction with local standard care pathways). PRONTO has two coprimary endpoints: initiation of intravenous antibiotics at 3 hours (superiority comparison) and 28-day mortality (non-inferiority comparison). The study has an internal pilot phase and group-sequential stopping rules for effectiveness and futility/safety, as well as a qualitative substudy and a health economic evaluation.
Ethics and dissemination
The trial protocol was approved by the Health Research Authority (HRA) and NHS Research Ethics Committee (Wales REC 2, reference 20/WA/0058). In England and Wales, the law allows the use of deferred consent in approved research situations (including ED studies) where the time dependent nature of intervention would not allow true informed consent to be obtained. PRONTO has approval for a deferred consent process to be used. Findings will be disseminated through peer-reviewed journals and presented at scientific conferences.
Trial registration number
ISRCTN54006056.
Keywords: accident & emergency medicine, infection control, adult intensive & critical care
Strengths and limitations of this study.
Sepsis has a problem with both over and under diagnosis, and a major strength of PROcalcitonin and NEWS2 evaluation for Timely identification of sepsis and Optimal (PRONTO) is the use of coprimary outcomes to assess effectiveness as an antimicrobial stewardship intervention but also to ensure safety which is vital for widespread clinical adoption of this intervention.
PRONTO is designed to integrate into routine UK clinical pathways and includes assessment of acceptability and practicality in emergency department settings.
Limitations of the study design include the intervention being a change in risk assessment rather than a formal prescribe/do not prescribe rule for antibiotic use, which could lead to higher rate of clinician preference in the study.
The use of deferred consent also has the potential to increase participant withdrawal from the trial, as not all patients would have agreed to prospective informed consent.
Introduction
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection1 and is a medical emergency requiring prompt antimicrobial therapy and physiological support. The identification, assessment and management of sepsis is challenging because of its many non-specific symptoms and signs, which can be caused by both infectious and non-infectious diseases. In line with international recommendations, the UK National Institute for Health and Care Excellence (NICE) sepsis guidelines suggest the administration of intravenous antibiotics within an hour in patients at risk of intensive care unit (ICU) admission and death.2 However, up to 50% of patients initially managed as sepsis in the emergency department (ED) do not have a final diagnosis of sepsis3 4 and often do not have an infection.5 6 The current approach leads to overuse of antibiotics with the associated risk of antimicrobial resistance, antibiotic-related adverse drug reactions (eg, Clostridium difficile infection)7 and extended hospital stays.
The challenge of delivering high-quality sepsis care in an ED setting has been well recognised.8 9 The third international consensus definition (Sepsis 3)1 recommended use of the quick Sequential Organ Failure Assessment (qSOFA) score, to identify patients at high risk of death and prolonged ICU stay. National Early Warning Score (NEWS) and NEWS2 are rapid physiology-based scoring systems which are used to detect and track the deteriorating patient. NEWS has been demonstrated to have better diagnostic accuracy to qSOFA in detection of severe outcomes in sepsis.10 11 However, with its higher sensitivity comes reduced specificity which can result in significant increased numbers of patients being managed as high risk for suspected sepsis with a corresponding pressure on ED departments. NEWS2 replaced NEWS scoring system as the standard monitoring tool in the National Health Service (NHS) in 201912 and has been found to be comparable or superior to NEWS.13–16 In October 2021, Surviving Sepsis Campaign recommended that immediate antibiotics (within 1 hour) should be targeted to those with septic shock and others with suspected sepsis could wait for up to 3 hours for initial assessment to target antimicrobial choice or identify non-infectious mimics.17
The emergence of COVID-19 has exacerbated this previously highlighted problem. COVID-19 is a viral infection which presents within the sepsis syndrome constellation. Secondary bacterial infections are uncommon at presentation to ED (3.5%),18 despite this up to 83% of patients with COVID-19 received antibiotics.19 20 NEWS2 scores are broadly predictive of COVID-19 outcome on presentation but does not appear to be predictive of bacterial coinfection.21 Initial investigations in the ED can be helpful in distinguishing between COVID-19 and bacterial pneumonia including typical radiographic change, and COVID-19 point-of-care diagnostics.8 These results would be available within 3 hours for assessment and could potentially reduce unnecessary antimicrobial usage in COVID-19 management.
Procalcitonin (PCT) is a reliable biomarker that changes early in the course of bacterial infection. A recent PCT is currently the biomarker with the most available evidence to identify bacterial infections and inform antibiotic prescription decisions. Cochrane meta-analysis9 demonstrated that the use of PCT to guide antibiotic treatment in patients with acute respiratory infections reduced antibiotic exposure and side effects, and improved survival. Nevertheless, while the US Food and Drug Administration (FDA) has approved PCT assays for use in sepsis, current UK NICE guidance does not recommend PCT use on the basis of insufficient evidence.22 23 PCT predictive of outcome in COVID-19, and this may be because of its ability to identify superadded bacterial infection.10 11 24 The available evidence suggests a low PCT will have good negative predictive value for a bacterial coinfection in cases of COVID-19.25
Aims and objectives
Primary objective
To assess whether the addition of PCT measurement to NEWS2 scoring leads to a reduction in intravenous antibiotic initiation at 3 hours, with no increase in 28-day mortality compared with NEWS2 scoring alone in the management of patients presenting to hospital EDs in England and Wales with suspected sepsis.
Secondary objective
The assessment of (1) feasibility, (2) cost-effectiveness and (3) acceptability to healthcare practitioners, patients and their family
Methods and analysis
Study design
PROcalcitonin and NEWS2 evaluation for Timely identification of sepsis and Optimal (PRONTO) is a multicentre, parallel two-arm, open-label, individually randomised controlled trial with two coprimary endpoints, an internal pilot phase and group-sequential stopping rules for effectiveness and futility/safety. Participants will be randomised in a ratio of 1:1 to standard clinical management based on NEWS2 scoring or standard clinical management based on NEWS2 scoring plus PCT-guided risk assessment.
Internal pilot
An internal pilot phase will be conducted over the first 9 months of the recruitment period with ten lead sites. Predefined progression criteria will be used to assess feasibility to progress to the full trial, such as site and patient absolute recruitment and consent rate, proportion of patients undergoing PCT assessments and the ability to collect coprimary outcome data.
Eligibility
Inclusion criteria
Up to 20 EDs from across England and Wales will recruit adults (≥16 years) who are being managed as suspected sepsis over a 24-month period. There is no minimum NEWS2 score for inclusion into the study.
Exclusion criteria
Patients already receiving intravenous antibiotics, currently receiving myeloablative chemotherapy, patients with solid-organ transplantation, allogeneic bone marrow or stem cell transplantation within 3 months prior to consent or patients known to require urgent surgical intervention at the time of randomisation.
Patients with an advance directive to withhold life-sustaining treatment or patients not wishing to receive cardiopulmonary resuscitation may qualify provided they receive all other resuscitative measures for example, respiratory support and fluid resuscitation.
Study procedures and progress
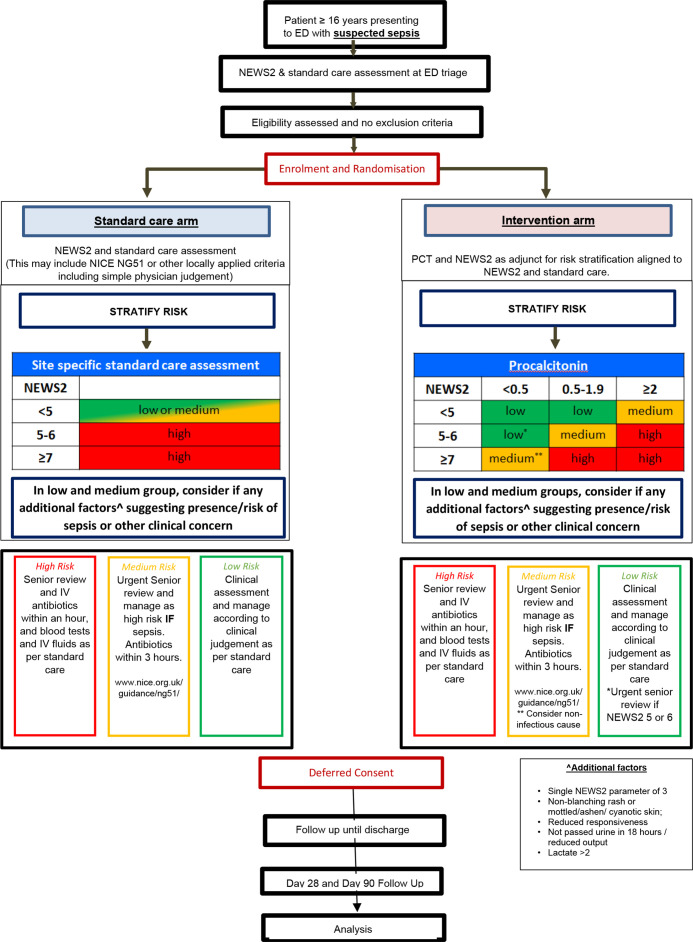
The trial schema is shown in figure 1.
Figure 1.
Trial schema. ED, emergency department; NEWS, National Early Warning Score; NICE, National Institute for Health and Care Excellence; PCT, procalcitonin.
The COVID-19 pandemic resulted in a delay to the original start date of June 2020. First participant was recruited on 20 November 2020. Current planned end date is 30 November 2022.
Identification and screening
Patients with suspected sepsis will be identified at ED triage. After initial NEWS2 scoring and assessment according to current standard of care the eligibility criteria will be assessed and if no exclusion criteria apply, patients will be enrolled into the trial and randomised. A screening log of all eligible and randomised patients will be kept at each site so that any biases from differential recruitment will be detected.
Randomisation
Participants will be individually randomised in a 1:1 ratio by delegated research staff within the ED to either to standard clinical management based on NEWS2 scoring (control) or standard clinical management based on NEWS2 scoring plus PCT-guided risk assessment (intervention). We will use minimisation with NEWS2 score (≥or < 5) and site as balancing factors and add a random element to reduce the risk of subversion.26 This will be implemented in a secure 24-hour web-based randomisation programme controlled centrally by the Centre for Trials Research (CTR) in Cardiff. Full details are provided in the PRONTO randomisation strategy.
Trial intervention
The BRAHMS PCT-direct reader (ThermoFisher Diagnostics (Altrincham, Cheshire, UK) is a fully validated, CE-marked point-of-care test to determine levels of PCT in the blood. The test requires 20 µL blood which will be obtained from either venous blood during standard care procedures at triage or via a finger-prick. This will be used in combination with NEWS2 assessment of adult patients with suspected sepsis in ED, using a guidance-only algorithm for clinicians (figure 1). The risk algorithm categorises individuals as low, medium or high risk, interpretation and management (table 1). Clinicians have oversight at all times as to whether to adhere to the algorithm As currently mandated in UK, NICE clinical guidelines and quality standard QS161,27 urgent senior review within an hour will take place should any healthcare provider identify at least one risk factor indicating high risk of progression to severe illness or death regardless of underlying aetiology. This equates to a NEWS2 ≥5 or an individual having a single feature of the evidence-based ‘NICE high-risk criterion’.
Table 1.
Clinical risk management interpretation
| Risk group | Interpretation |
| High | High risk of progression to sepsis. Likely benefit from immediate antibiotics (within 1 hour) |
| Medium | Medium risk of progression to sepsis. likely benefit from early antibiotics (within 3 hours) but consider non-bacterial sources and likely source. Allows clinical teams time to complete rapid assessment In patients with high NEWS2 but low PCT (<0.5) explicit advice to consider non-infectious causes of presentation |
| Low | Low risk of progression to sepsis. consider non-bacterial sources, likely source and whether requires antibiotics |
NEWS2, National Early Warning Score 2; PCT, procalcitonin.
Informed consent
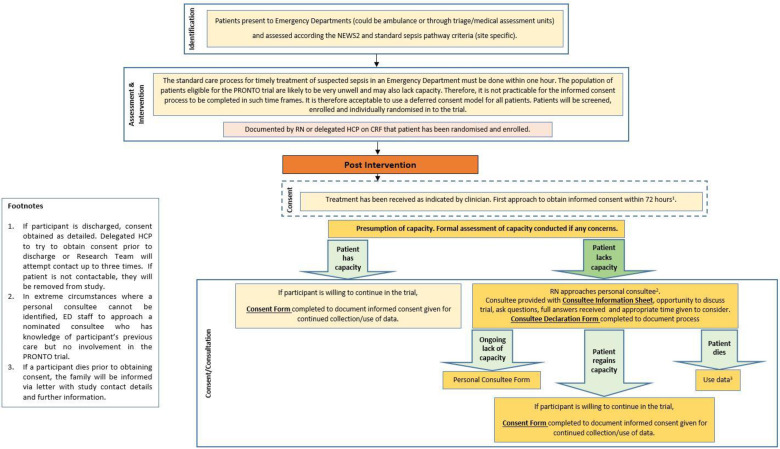
Research carried out in emergency situations is challenging in terms of obtaining consent. Emergency research is when treatment needs to be given urgently, and it is necessary to take urgent action for the purposes of the study. In some emergency situations people may lack capacity to give consent themselves and obtaining consent from a legal representative or consulting others is not reasonably practicable. In England and Wales, the law allows adults who lack capacity to take part in emergency research without prior consent from a legal representative or consulting others, if certain conditions are met (Medicines for Human Use (Clinical Trials) Amendment (No 2) Regulations SI 2006 2984, Mental Capacity Act s32).28 Given the requirement for rapid clinical assessment and treatment in the management of suspected sepsis, for this trial we will use a deferred consent model. Patients and their relatives will be informed that a study is ongoing but a lengthy consent discussion will not be had so as not to delay treatment. Should the patient or consultee wish not to take part at this point, then the decision will be respected and the patient will not be enrolled into the trial. Following randomisation an approach to obtain informed consent will be made as soon as is practicably feasible, ideally within 72 hours (figure 2). Where a participant lacks mental capacity, a maximum of three approaches will be made. After three approaches, or if the participant is not likely to regain mental capacity, a personal consultee will be approached. In extreme circumstances, where no personal consultee can be identified, a nominated consultee will be approached. Separate informed consent will be taken for participation in the qualitative data collection. Patients who do not consent to continue in the study will be withdrawn completely from the study. A tiered consent model is used in this study and allows participants to consent to different aspects of the study (online supplemental appendix table 1). An example participant consent form is available in online supplemental appendix.
Figure 2.
Consent procedures. CRF, case report form; ED, emergency department; HCP, Health Care Professional; NEWS, National Early Warning Score; PRONTO, PROcalcitonin and NEWS2 evaluation for Timely identification of sepsis and Optimal use of antibiotics in the emergency department; RN, Research Nurse.
bmjopen-2022-063424supp001.pdf (1.2MB, pdf)
Data collection during primary admission
All data collection will be by electronic data capture using a bespoke database developed by the CTR and hosted by Cardiff University secure servers. It is encrypted and accessed by individual username and password. Paper copies of all case report forms will be available. Essential documents will be kept securely in a locked cupboard, and at the end of the trial, will be archived at an approved external storage facility for 10 years. A member of the research team in ED will undertake the data collection relating to the NEWS2 screening, trial intervention and whether clinical teams followed the intervention or standard of care risk assessment. Participants who consent to continue in the study will have daily information collected from the date of randomisation until they are discharged from hospital or until day 28, whichever is sooner. Trial data is collected from patients’ health records and no trial visits occur between consent and day 28. Key follow-up data are listed in online supplemental appendix table 2.
Follow-up
Twenty-eight-day follow-up
Day 28 follow-ups will be conducted via telephone or in person if the participant remains an inpatient. These will comprise a European Quality of life five dimension, five level (EQ-5D/5L) validated questionnaire for participant or proxy completion, and a Health Economics questionnaire where patient outcomes (readmission, retreatment, hospital-acquired infection) and use of healthcare resource (hospital admissions, outpatient parenteral antimicrobial therapy, other prescribed medicines, privately purchased over-the-counter medicines, General Practitioner (GP) and hospital outpatient attendance) will be captured. In addition, direct non-medical costs borne by patients/carers as a result of attending hospital (travel costs, childcare costs, expenses incurred while in hospital, self-reported lost earnings and other direct non-medical expenses) will be collected.
Ninety-day follow-up
EQ-5D/5L questionnaires will be repeated and a shortened Health Economics questionnaire to capture any additional costs or hospital admissions since the day 28 questionnaires will be completed.
Withdrawal
Participants have the right to withdraw from the study at any time and can request that all data collected up to that point is not used.
Safety and pharmacovigilance
The trial population comprises unwell hospital inpatients. Events such as prolongation of existing hospitalisation, life threatening events and death are expected in this population and are recorded as part of routine data collection and therefore are not subject to expedited reporting. Serious adverse events will be reported if the event results in persistent or significant disability or incapacity or consists of a congenital anomaly or birth defect. An assessment of causality between the event and the trial intervention will be carried out by the principal investigator or delegated clinician, and then independently by a clinical reviewer. If the clinical reviewer classifies the event as probably or definitely caused by the intervention, it will be classified as a serious adverse reaction. Non-serious Adverse Events (AEs) potentially attributable to the PCT test will be collected as part of routine follow-up at 28 days. Any other non-serious AEs will not be collected.
Data management
Details of data management procedures (such as checking for missing, illegible or unusual value (range checks) will be specified in the PRONTO Data Management Plan. Details of Monitoring procedures will be specified in the PRONTO Monitoring plan.
Statistical analysis
Outcome measures
The coprimary outcomes of this study are the initiation of intravenous antibiotics at 3 hours (intervention arm to be shown superior to control) and 28-day mortality (intervention arm to be shown non-inferior to control). Coprimary and secondary outcomes are listed in box 1. Final decisions about the primary effectiveness of the intervention, using these coprimary outcomes will be made based on the decision matrix (table 2). All outcomes will be stratified by COVID-19 diagnosis (SARS-CoV2 PCR positive or high likelihood of clinical COVID-19 as determined by a senior clinician).
Box 1. Coprimary and secondary outcomes.
Coprimary outcome measures:
Intravenous antimicrobial initiation—binary outcome assessed at 3 hours hours.
Twenty-eight-day mortality—binary outcome.
Secondary outcome measures:
Time until initiation of intravenous antibiotic therapy.
Late intravenous antibiotic initiation—antibiotics commenced after 3 hours hours.
Number of days on intravenous antibiotics (during admission and total over the first 28 days).
Number of days on any antibiotic (during admission and total over the first 28 days).
Number of days on broad spectrum antibiotics (intravenous and oral), defined by number of days on an Access group of antibiotics as defined by WHO AWaRe Classification Database (during admission and total over the first 28 days).
Intensive care unit (ICU) admission intravenous at any point during admission.
Length of ICU stay.
Length of hospital stay.
Adverse antibiotic outcomes.
Readmission to hospital within 90 days.
Mortality within 90 days (and time until death).
Health utility (European Quality of life five dimension, five level (EQ-5D/5L)) at 28 and 90 days.
Health resource usage.
Feasibility of implementing procalcitonin (PCT) testing alongside National Early Warning Score 2 (NEWS2) scoring in emergency departments (EDs).
Acceptability of implementing PCT testing alongside NEWS2 scoring in EDs, to patients, carers and clinicians.
Total average cost per patient per arm and cost per gained (health-adjusted) life year.
Table 2.
Decision matrix for coprimary outcomes
| Reduced antibiotic initiation | Same or more antibiotic initiation | |
| Decreased mortality | Effective | Effective |
| Equivalent mortality | Effective | Not effective |
| Increased mortality | Not effective/harmful | Not effective/harmful |
Sample size
The sample size calculation is based on two coprimary outcomes:29
Twenty-eight-day mortality, for which we want to show non-inferiority of the PCT guided assessment as compared with current standard practice, using an absolute 2.5% non-inferiority margin. Assuming a 28-day mortality of 15% in patients managed as suspected sepsis treated in the ED,3 30 this means that any increase in 28-day mortality from 15% to not more than 17.5% would be considered non-inferior. For 90% power and one-sided 5% significance level the sample size required is 7002, assuming there is no difference in 28-day mortality between arms. Our patient focus group were also consulted on the 2.5% non-inferiority margin and felt that this was acceptable if there were mechanisms to monitor trial outcomes, and if this was what was needed to provide a sample size which would ensure the trial could be completed as well as answer the research question.
Initiation of antibiotics treatment, for which we want to show superiority. Currently around 90% of patients managed as suspected sepsis receive antibiotics (Liverpool University Hospitals NHS Foundation Trust, unpublished data). A reduction to 80% would be considered a success. To detect such an effect with 90% power and two-sided 5% significance level the sample size required is 532, which is substantially lower than what is needed for the non-inferiority endpoint. With 7002 patients we would be able to detect effects as small as a reduction from 90% to 87.6%, with 90% power.
Accounting for 5% drop-out, we would need a total sample size of 7372. The group-sequential design with O’Brien-Fleming stopping boundaries for both effectiveness and futility/safety will increase the total maximum sample size (if the study is not stopped after the interim analysis) by just over 4% to 7676 (inflated for 5% drop-out).
These sample sizes were calculated using SAS V.9.4 PROC POWER and PROC SEQDESIGN.
Interim analysis
A planned interim analysis of the coprimary outcomes will be conducted when 50% of patients have been recruited and followed up for 28 days. Stopping the study shall be recommended by the independent data monitoring committee (IDMC) based on group-sequential O’Brien-Fleming boundaries. They shall recommend stopping for effectiveness if:
The PCT-guided assessment is superior in terms of 28-day mortality (ie, a significant reduction to less than 15%).
The PCT-guided assessment is non-inferior in terms of 28-day mortality and superior in terms of initiation of antibiotics.
They shall recommend stopping for futility if the results of the interim analysis suggest futility for both endpoints. This strategy ensures overall type I error rate control.31 32 The exact stopping rules will be specified in an interim analysis plan.
Final analysis
The primary analysis will be intention to treat and will fit separate two-level logistic regression models (patients nested within sites) to both coprimary outcomes (antibiotic initiation and mortality), controlling for baseline NEWS2 score (minimisation factor). The intervention will be considered effective if there is both a significant reduction in antibiotic initiation (two-sided 5% level) and if the difference in mortality between the two groups is non-inferior (one-sided 5% level). In case the 28-day mortality rate in the control arm deviates from the assumed 15%, the absolute 2.5% non-inferiority margin will be replaced with an arcsine difference ‘non-inferiority frontier’.33 The primary analysis will be adjusted to account for the group-sequential design. Imputation of missing data will be done as part of sensitivity analyses.
In a secondary analysis, complier adjusted causal effect models will be fitted to allow for non-adherence to the intervention. Two models will be fitted allowing for two different definitions of adherence:
Patients randomised to PCT-guided care in whom a PCT test is done and the clinician considers the results as part of their decision making.
Patients randomised to PCT-guided care in whom a PCT test is done and the clinician follows the algorithm exactly.
Analyses of secondary outcomes will also be performed as intention to treat and using appropriate two-level regression models depending on the type of outcome (eg, linear regression for continuous outcomes, Cox regression for time-to-event outcomes) to allow for patients nested within sites. This includes an HTA and economic evaluation as per CHEERS 2022 guidance. Analyses will be split by organ system of the infection (eg, lower urinary tract, lower respiratory, intra-abdominal, bacteraemia, skin and soft tissue). Stratified analyses will be undertaken at different levels of NEWS2 scoring ≤4, 5–6 and ≥7, and will also be undertaken by COVID-19 status. All further details will be specified in a statistical analysis plan which will be finalised prior to database lock for the planned interim analysis and subsequently published.
Missing primary outcome data are likely to be minimal, so complete-case analysis will be used. However, if this exceeds more than 20% of participants we will employ multiple imputation and report the impact on the treatment effect alongside the complete-case analysis.
Qualitative study
The qualitative work will have three components: interviews with clinicians, interviews with patients/carers, and observations of trial implementation (when appropriate during the ongoing current COVID-19 pandemic). Findings will be used to aid understanding of the quantitative data and provide areas for improvement in processes to enhance the efficiency of the trial.
Interviews with clinicians will take place at two time points. Interview 1 will take place during the pilot phase and will be a semistructured interview with 10–12 clinicians at <5 study sites (2–3 per site). This will explore the feasibility and acceptability of research processes and integration of the PCT algorithm into their ED setting. Interview 2 will be with clinicians towards the end of the trial when they have more experience of using the PCT algorithm and will identify barriers and facilitators to the use of the PCT test and algorithm in more detail, including reasons for deviating from the study algorithm.
We will conduct semistructured interviews with patients after the 90-day follow-up, in order to gain a detailed understanding of patients’ experiences of care to aid understanding of trial results. We will encourage patients to include a close family member in the interview also. This will allow us to capture an additional perspective on the patients’ care.
Patient and public involvement
The proposal has benefited from multiple interactions with patient and public involvement (PPI) groups to refine the research question and design. Author JC is a lay coapplicant/patient representative, who has coproduced and helped finalise the study design. As a coapplicant JC is a member of the trial management group (TMG) ensuring that all patient facing materials are presented in a suitable way. Her experience is invaluable throughout the project, including the promotion of the trial to potential participants and appropriate dissemination of findings to the lay public.
In addition, we have convened wider PPI advisory panels from both higher education institutions and NHS patient groups. We discussed the trial with the panel at the Royal Liverpool Hospital in August 2018, focusing on need, conception, design and trial management. The group fully supported the need for this trial recognising the potential for PCT measurement to improve outcomes for patients with suspected sepsis and supported the use of deferred consent. Specific feedback about these aspects has now been used to update the relevant parts of the proposal.
Trial management
The trial is sponsored by the University of Liverpool and coordinated by Cardiff University CTR.
Trial management group
The TMG will meet monthly throughout the course of the trial and will include the cochief investigators, coapplicants, collaborators, trial manager, data manager and administrator. TMG members will be required to sign up to the remit and conditions as set out in the TMG charter.
Trial steering committee and IDMC
An independent trial steering committee (TSC) consisting of an independent chairperson, two independent members and a patient representative will provide oversight of the PRONTO trial. There will also be a separate IDMC to provide oversight of all matters relating to patient safety and data quality, and recommend continuing or stopping the trial depending on the results of the interim analysis. Members will be required to sign up to the remit and conditions as set out in the TSC and IDMC charters and will meet at least annually.
Ethics and dissemination
Ethics approvals and consent
The trial was approved by the NHS Research Ethics Committee (Wales REC 2, reference 20/WA/0058) on the 21 July 2020 and subsequent Health Research Authority (HRA) and Health and Care Research Wales approval was granted on 22 July 2020. In England and Wales, the law allows the use of deferred consent in approved research situations (including ED studies) where the time dependent nature of intervention would not allow true informed consent to be obtained. PRONTO has approval for a deferred consent process to be used, full details are in Informed Consent section above. The following substantial amendments were made to the trial and were communicated to all trial sites: Amendment 5 (23 October 2020); Amendment 7 (10 December 2020); Amendment 9 (25 February 2021); Amendment 12 (29 June 2021), Amendment 15 (15 October 2021), Amendment 17 (6 January 2022).
Dissemination plan
We will engage with patient groups and the wider public through relevant charities such as UK Sepsis Trust and Antibiotics Action, and seek to present trial updates at their annual conferences. We will use press releases and social media outlets to publicise the trial and disseminate findings. A 90 s animation outlining the PRONTO main aims was commissioned https://www.youtube.com/watch?v=H3x-rNVlwJI 34 and accessed via posters and patient information leaflets via a scannable QR code. At the end of the trial, a final report will be prepared for the National Institute of Health Research Health Technology Assessment Journal series. The results will be disseminated locally, nationally and internationally among scientific, clinical and lay groups including participants and their families. All publications and presentations related to the trial will be authorised by the TMG in accordance with the PRONTO publication policy. Where appropriate, the results of this trial can be directly implemented in the revisions of the NICE guidelines.
Supplementary Material
Acknowledgments
In addition to the authors, the PRONTO team comprises Sam Clarkstone, Jacqui Hughes and Lena Meister. The authors would like to pay special thanks to Principal Investigators Dr Andrew Tabner (Royal Derby Hospital) and Dr Gavin Barlow (Hull Royal Infirmary) for their input and constructive feedback at PRONTO TMG meetings. The authors would like to thank the research nurses and clinicians for their support during the trial. They would also like to acknowledge the contribution of the TSC members, namely Professor Timothy Peto, Professor Richard Body, Mike Bradburn, Professor Jane Daniels, Emma Richards and Angela Brain. The authors would also like to acknowledge the contribution of the IDMC members, namely Professor Sarah Walker, Professor Steve Goodacre, Dr Matthew Scarborough, Sian O'Shea and Claire James.
Footnotes
Twitter: @Dr_JoanneEuden, @emma_tj1, @stephen_aston, @CarrolEnitan
Contributors: NF and ST are co-chief investigators of this trial. NF and ST, along with ET-J, EC, LB-H, PH, MI-K, ML, FM, LWN, EN, PP, PS, DT-R, IW and KH led the development of the research question, study design, obtaining the funding and implementation of the protocol. JE is the Trial Manager and ET-J is the senior trial manager who coordinate the operational delivery of the trial protocol and recruitment. LB-H is the lead qualitative researcher. PP is the trial statistician. SG is the data manager. All authors listed provided critical review and final approval of the manuscript.
Funding: This trial is funded by the National Institute of Health Research Health Technology Assessment (NIHR HTA) programme, funder reference 17/136/13. The Centre for Trials Research at Cardiff University receives infrastructure funding from Health and Care Research Wales. The study is supported by the NIHR Clinical Research Network.
Disclaimer: The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. Neither the Sponsor nor the Funder had any role in the study design; collection, management, analysis and interpretation of data; writing of this manuscript or in the decision to submit this manuscript for publication. ThermoFisher are not funding the study, and have no influence on the design, conduct or reporting of the study
Competing interests: EC is co-CI for the BATCH Trial (HTA 15/188/42) and the PEACH study (HTA Project: NIHR132254) on PCT use, and member of NICE Diagnostic advisory committee (2014–2020), and NICE Sepsis guideline development committee (2014-6). All other authors declare no competing interests.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Patient and public involvement section for further details.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
the PRONTO team:
Sam Clarkstone, Jacqui Hughes, Lena Meister, Andrew Tabner, Gavin Barlow, Richard Body, Mike Bradburn, Jane Daniels, Emma Richards, Angela Brain, Sarah Walker, Steve Goodacre, Matthew Scarborough, Sian O'Shea, and Claire James
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Singer M, Deutschman CS, Seymour CW, et al. The third International consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:801–10. Online First: Epub Date. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. NICE Sepsis Guide Development Group . Sepsis: recognition, diagnosis and early management, NICE guideline [NG51]. Available: LastUpdate.www.nice.org.uk/guidance/ng51
- 3. Corfield AR, Lees F, Zealley I, et al. Utility of a single early warning score in patients with sepsis in the emergency department. Emerg Med J 2014;31:482–7. 10.1136/emermed-2012-202186 [DOI] [PubMed] [Google Scholar]
- 4. Burston J, Adhikari S, Hayen A, et al. A role for antimicrobial stewardship in clinical sepsis pathways: a prospective interventional study. Infect Control Hosp Epidemiol 2017;38:1032–8. 10.1017/ice.2017.139 [DOI] [PubMed] [Google Scholar]
- 5. Singer M, Inada-Kim M, Shankar-Hari M. Sepsis hysteria: excess hype and unrealistic expectations. Lancet 2019;394:1513–4. 10.1016/S0140-6736(19)32483-3 [DOI] [PubMed] [Google Scholar]
- 6. Klein Klouwenberg PMC, Cremer OL, van Vught LA, et al. Likelihood of infection in patients with presumed sepsis at the time of intensive care unit admission: a cohort study. Crit Care 2015;19. 10.1186/s13054-015-1035-1. [Epub ahead of print: Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O'Neill J. Tackling drug-resistant infections globally: final report and recommendations. London: The review on antimicrobial resistance, 2016. [Google Scholar]
- 8. Goodacre S, Thomas B, Lee E, et al. Characterisation of 22445 patients attending UK emergency departments with suspected COVID-19 infection: observational cohort study. PLoS One 2020;15. 10.1371/journal.pone.0240206. [Epub ahead of print: Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schuetz P, Wirz Y, Sager R, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis 2018;18:95–107. 10.1016/S1473-3099(17)30592-3 [DOI] [PubMed] [Google Scholar]
- 10. Ou M, Zhu J, Ji P, et al. Risk factors of severe cases with COVID-19: a meta-analysis. Epidemiol Infect 2020;148. 10.1017/S095026882000179X. [Epub ahead of print: Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tjendra Y, Al Mana AF, Espejo AP, et al. Predicting disease severity and outcome in COVID-19 patients: a review of multiple biomarkers. Arch Pathol Lab Med 2020;144:1465–74. 10.5858/arpa.2020-0471-SA [DOI] [PubMed] [Google Scholar]
- 12. RCo P. National early warning score (news) 2. Available: https://www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2 [Accessed Last Update December 2017].
- 13. Graham CA, Hung KKC, Leung LY. Sirs and NEWS2 to predict 60-Day mortality in the emergency department: prospective study. Am J Respir Crit Care Med 2019. 10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A7157 [DOI] [Google Scholar]
- 14. Mellhammar L, Linder A, Tverring J, et al. NEWS2 is superior to qSOFA in detecting sepsis with organ dysfunction in the emergency department. J Clin Med 2019;8. 10.3390/jcm8081128. [Epub ahead of print: 29 07 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Churpek MM, Snyder A, Han X, et al. Quick sepsis-related organ failure assessment, systemic inflammatory response syndrome, and early warning scores for detecting clinical deterioration in infected patients outside the intensive care unit. Am J Respir Crit Care Med 2017;195:906–11. 10.1164/rccm.201604-0854OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Redfern OC, Smith GB, Prytherch DR, et al. A comparison of the quick sequential (sepsis-related) organ failure assessment score and the National early warning score in Non-ICU patients with/without infection. Crit Care Med 2018;46:1923–33. 10.1097/CCM.0000000000003359 [DOI] [PubMed] [Google Scholar]
- 17. Evans L, Rhodes A, Alhazzani W, et al. Executive summary: surviving sepsis campaign: international guidelines for the management of sepsis and septic shock 2021. Crit Care Med 2021;49:1974–82. 10.1097/CCM.0000000000005357 [DOI] [PubMed] [Google Scholar]
- 18. Sze S, Pan D, Williams CML, et al. Letter to the editor: variability but not admission or trends in NEWS2 score predicts clinical outcome in elderly hospitalised patients with COVID-19. J Infect 2021;82:159–98. 10.1016/j.jinf.2020.05.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect 2020;26:1622–9. 10.1016/j.cmi.2020.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020;369. 10.1136/bmj.m1985. [Epub ahead of print: Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Myrstad M, Ihle-Hansen H, Tveita AA, et al. National Early Warning Score 2 (NEWS2) on admission predicts severe disease and in-hospital mortality from Covid-19 - a prospective cohort study. Scand J Trauma Resusc Emerg Med 2020;28. 10.1186/s13049-020-00764-3. [Epub ahead of print: Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. NICE . Procalcitonin testing for diagnosing and monitoring sepsis (ADVIA Centaur BRAHMS PCT assay, BRAHMS PCT Sensitive Kryptor assay, Elecsys BRAHMS PCT assay, LIAISON BRAHMS PCT assay and VIDAS BRAHMS PCT assay): Daignostic Guidance [DG18]; 2015.
- 23. Westwood M, Ramaekers B, Whiting P, et al. Procalcitonin testing to guide antibiotic therapy for the treatment of sepsis in intensive care settings and for suspected bacterial infection in emergency department settings: a systematic review and cost-effectiveness analysis. Health Technol Assess 2015;19:1–236. 10.3310/hta19960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Richards O, Pallmann P, King C, et al. Procalcitonin increase is associated with the development of critical care-acquired infections in COVID-19 ARDS. Antibiotics 2021;10. 10.3390/antibiotics10111425. [Epub ahead of print: 22 Nov 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kamat IS, Ramachandran V, Eswaran H, et al. Procalcitonin to distinguish viral from bacterial pneumonia: a systematic review and meta-analysis. Clin Infect Dis 2020;70:538–42. 10.1093/cid/ciz545 [DOI] [PubMed] [Google Scholar]
- 26. Altman DG, Bland JM. Treatment allocation by minimisation. BMJ 2005;330. 10.1136/bmj.330.7495.843. [Epub ahead of print: Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. NIHR . Sepsis: Quality standard [QS161]. Available: https://www.nice.org.uk/guidance/qs161
- 28. Medicines for human use (Clinical trials) Amendment Regulations (SISi 2006/1928) 2006.
- 29. Gillespie D, Francis NA, Carrol ED, et al. Use of co-primary outcomes for trials of antimicrobial stewardship interventions. Lancet Infect Dis 2018;18:595–7. 10.1016/S1473-3099(18)30289-5 [DOI] [PubMed] [Google Scholar]
- 30. Goulden R, Hoyle M-C, Monis J, et al. qSOFA, SIRS and news for predicting inhospital mortality and ICU admission in emergency admissions treated as sepsis. Emerg Med J 2018;35:345–9. 10.1136/emermed-2017-207120 [DOI] [PubMed] [Google Scholar]
- 31. Hamasaki T, Asakura K, Evans SR, et al. Group-Sequential strategies in clinical trials with multiple co-primary outcomes. Stat Biopharm Res 2015;7:36–54. 10.1080/19466315.2014.1003090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Flight L, Julious SA. Practical guide to sample size calculations: non-inferiority and equivalence trials. Pharm Stat 2016;15:80–9. 10.1002/pst.1716 [DOI] [PubMed] [Google Scholar]
- 33. Quartagno M, Walker AS, Babiker AG, et al. Handling an uncertain control group event risk in non-inferiority trials: non-inferiority frontiers and the power-stabilising transformation. Trials 2020;21:145. 10.1186/s13063-020-4070-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Animated S. Optimising antibiotic use in sepsis: PRONTO trial. Available: https://www.youtube.com/watch?v=H3x-rNVlwJI
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-063424supp001.pdf (1.2MB, pdf)