ABSTRACT
The U.S. Centers for Disease Control and Prevention (CDC) and other health agencies have recently recommended a booster dose of COVID-19 vaccines for specific vulnerable groups including adults 65 years and older. There is limited evidence whether vaccine effectiveness (VE) in older adults decreases over time, especially against severe COVID-19. We performed a rapid review of published studies available through 4 November 2021 that provide effectiveness data on messenger RNA (mRNA) vaccines approved/licensed in the United States and identified eight eligible studies which evaluated VE in older adults. There is evidence of a decline in VE against both severe acute respiratory syndrome coronavirus 2 infection and severe COVID-19 in older adults among studies which analyzed data up to July–October 2021. Our findings suggest that VE diminishes in older adults, which supports the current recommendation for a booster dose in this population.
KEYWORDS: Vaccine, COVID-19, waning, older adults, effectiveness
Introduction
Authorized/licensed COVID-19 mRNA vaccines had shown remarkable efficacy and real-world effectiveness against both asymptomatic and symptomatic disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) including in adults aged 65 or older.1,2 However, accumulating evidence suggests that vaccine effectiveness (VE) against SARS-CoV-2 infection declines over time in some groups, including older adults who have the highest risk of morbidity and mortality from COVID-19.3 This waning effectiveness, along with the emergence of variants of concern (VOC), may be associated with a resurgence of COVID-19 cases.4,5 On 22 September 2021, the Food and Drug Administration (FDA) granted Emergency Use Authorization (EUA) for booster doses of the Pfizer BioNtech mRNA vaccine in specific populations including older adults 65 years and older; long-term care setting residents; those who have high risk of severe COVID-19; and those who work or live in high-risk settings.6,7 On 20 October 2021 a similar EUA was granted regarding Moderna boosters. There are relatively few published studies regarding the durability of SARS-CoV-2 mRNA VE in older adults with respect to protection against severe COVID-19 leading to hospitalization and death.
Several studies have reported VE of older adults against SARS-CoV-2 infection and severe COVID-19. Each study varies in terms of vaccines, geographical regions or countries, and observation period after immunization. Each of these factors may potentially affect generalizability of findings to other settings. Furthermore, dominant circulating strains of SARS-CoV-2 during the study period may vary between studies. To the best of our knowledge, there is no comprehensive review to assess waning VE in older adults, representing a knowledge gap with important implications for vaccination policy. We therefore reviewed and synthesized available data of VE among older adults for currently approved/licensed mRNA vaccines in the USA (i.e. BNT162b2 and mRNA1283) from peer-reviewed published PubMed-indexed studies that were available through 4 November 2021. Aiming to enhance the evidence base informing public health policy, we assessed VE in adults stratified by study period and with an emphasis on the older age group.
Methods
We conducted a PubMed search on 4 November 2021 for English-language articles, using combinations of the search terms of “SARS-CoV-2”, “COVID-19”, “vaccine”, “effectiveness”, “efficacy”, “older adults”, and “elder”. We also searched reference lists of identified articles and other relevant articles on effectiveness of COVID-19 vaccines in older adults. We included human studies which evaluated VE of the two mRNA vaccines employed in the U.S. (BNT162b2 and mRNA1273) in adults aged 65 or older. Given considerable variation between countries in key factors such as timing of vaccine campaigns, modification of vaccine regimens (e.g. prolonged interval between immunizations), and circulating SARS-CoV-2 strains, we limited our review to U.S.-based studies. We excluded preprint papers and studies which only report VE after single dose, and only considered estimates of VE at least seven days after second vaccination. In this review, the term VE refers to protection from outcomes such as infection, symptomatic illness, hospitalization, and death; it does not refer to circulating levels of neutralizing antibodies.
We captured three key elements from each eligible study: (1) the definition and estimate of VE; (2) the observation period; and (3) the number of participants. We also captured VE estimates for younger adults if included in the same article. We classified each study definition of VE as protection from any SARS-CoV-2 infection versus protection from severe COVID-19 defined as hospital admission or death. We defined three calendar periods based on phase of pandemic and emergence of the delta variant of SARS-CoV-2: ‘early pandemic’ through 8 May 2021, when the rate of delta variant infection was ≤1%; ‘mid pandemic’ between 9 May and 30 June 2021, when incidence of delta variant infection increased; and ‘late pandemic’ from 1 July 2021, when delta was the predominant strain.
Results
After screening titles, abstracts, and full-text reviews, we identified a final set of N = 8 published articles that met our eligibility criteria. All articles evaluated VE of adults ≥65 years of age, while six of eight (88%) studies also analyzed VE in younger adults. One study evaluated VE for BNT162b2 vaccine, while six evaluated VE for BNT162b2 and mRNA1273 vaccines either separately or combined. Four studies (papers 1–4) analyzed VE exclusively during the early pandemic period. Three studies (papers 5–7) analyzed VE during the mid pandemic period: these papers included some data from the early or late pandemic period. One study (paper 8) analyzed VE exclusively during the late pandemic period when delta was the predominant circulating variant (Table 1 and Figure 1).
Table 1.
Characteristics of the eligible papers
| Paper | Authors | Vaccine | Design | Study Period | Definition of VE | Age groups (year) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Young-Xu Y et al. | BNT162b2 and mRNA1273 | Test-negative study | 14 December 2020 to 14 March 2021 | SARS-CoV-2 infection (lab-confirmed, regardless of symptoms) ≥14 days after 2nd dose | 18–64 and ≥65 | 8 |
| 2 | Tenforde MW et al. (#1) | BNT162b2 and mRNA1273 | Test-negative study | 1 January 2021 to 26 March 2021 | Hospitalization due to COVID-19 ≥ 14 days after 2nd dose | ≥65 | 9 |
| 3 | Moline HL et al. | BNT162b2 and mRNA1273 | Observational cohort study | 1 February 2021 to 30 April 2021 | Hospitalization due to COVID-19 ≥ 14 days after 2nd dose | 65–74, and ≥75 | 10 |
| 4 | Tenforde MW et al. (#2) | BNT162b2 and mRNA1273 | Test-negative study | 11 March 2021 to 5 May 2021 | Hospitalization due to COVID-19 ≥ 14 days after 2nd dose | 18–49, 50–64, and ≥65 | 11 |
| 5 | Thompson MG et al. | BNT162b2 and mRNA1273 | Test-negative study | 1 January 2021 to 22 June 2021 | Hospitalization due to COVID-19 ≥ 14 days after 2nd dose | 50–64, 65–74, 75–84 and ≥85 | 12 |
| 6 | Bajema KL et al. | BNT162b2 and mRNA1273 | Test-negative study | 1 February 2021 to 6 August 2021 | Hospitalization due to COVID-19 ≥ 14 days after 2nd dose | 18–64 and ≥65 | 13 |
| 7 | Tartof SY et al. | BNT162b2 | Observational cohort study | 14 December 2020 to 8 August 2021 | SARS-CoV-2 infection (lab-confirmed, regardless of symptoms), or hospitalization due to COVID-19 ≥ 7 days after 2nd dose | 16–44, 45–64, and ≥65 | 14 |
| 8 | Cohn BA et al. | BNT162b2 and mRNA1273 | Observational cohort study | 1 July 2021 to 31 October 2021 | Death due to COVID-19 ≥ 14 days after 2nd dose | 18–64 and ≥65 | 15 |
Figure 1.
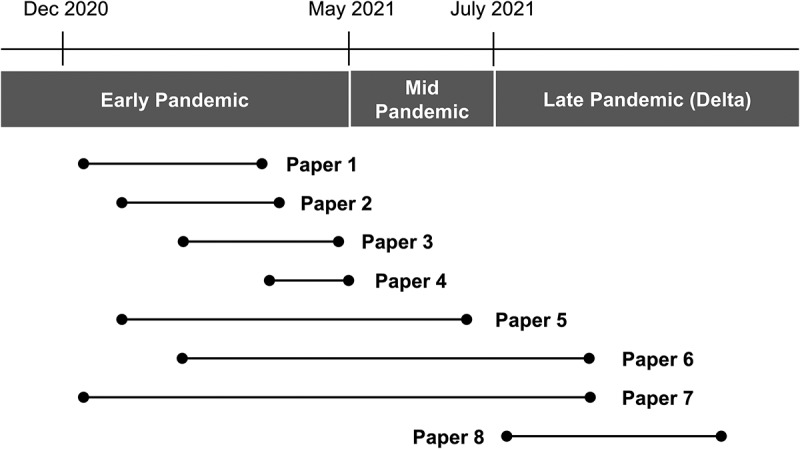
Study period of each eligible study.
Three calendar periods were defined based on phase of pandemic and emergence of the delta variant of SARS-CoV-2: ‘early pandemic’ through 8 May 2021, when the rate of delta variant infection was ≤1%; ‘mid pandemic’ between 9 May and 30 June 2021, when incidence of delta variant infection increased; and ‘late pandemic’ from 1 July 2021, when delta was the predominant strain. Each horizontal line represents study period of each paper.
VE against SARS-CoV-2 infection among older adults
Two studies (papers 1 and 7) examined mRNA VE against SARS-CoV-2 infection among older adults (Table 1).8,14 One early study (paper 1) evaluated VE between 14 December 2020 and 14 March 2021, reporting that mRNA vaccines demonstrated VE of 93% among older adults against SARS-CoV-2 infection (Figure 2).8 In contrast, a study (paper 7) which evaluated VE during 14 December 2020 and 8 August 2021 showed considerable decline of VE against SARS-CoV-2 infection to 61% (Figure 2).14
Figure 2.
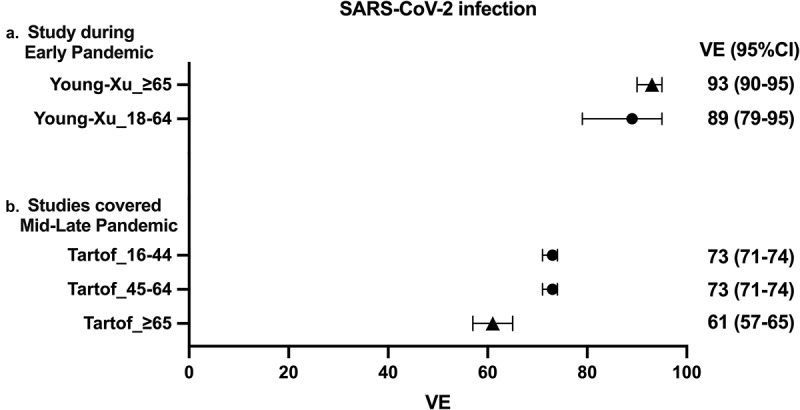
Forest plots of the VE against SARS-CoV-2 infection.
Point estimates and confidence intervals are depicted for mRNA VE against SARS-CoV-2 infection. VE for BNT162b2 and mRNA1273 were evaluated at least seven days after the second vaccination. Circle symbols represents data for young adults (<65 years of age) and triangle symbols represents data for older adults (≥65 years of age). VE: vaccine effectiveness, CI: confidence interval.
VE against severe COVID-19 among older adults
Seven studies examined mRNA VE against severe COVID-19 among older adults (Table 1).9–15 Among the three early studies (papers 2–4), estimates of VE against severe COVID-19 ranged from 87.3% to 96.8%.9–11 Among three studies (papers 5–7) which evaluated VE up to 8 August 2021, estimates of VE ranged from 80% to 90% among older adults (Figure 3).12–14 One study (paper 8) evaluated VE of mRNA vaccines among US veterans during 01 July to 31 October 202115. The study evaluated VE against severe COVID-19 defined as death and reported marked decline in VE for older adults ranging from 70.1% (for BNT162b2) to 75.5% (for mRNA1273) (Figure 3).
Figure 3.
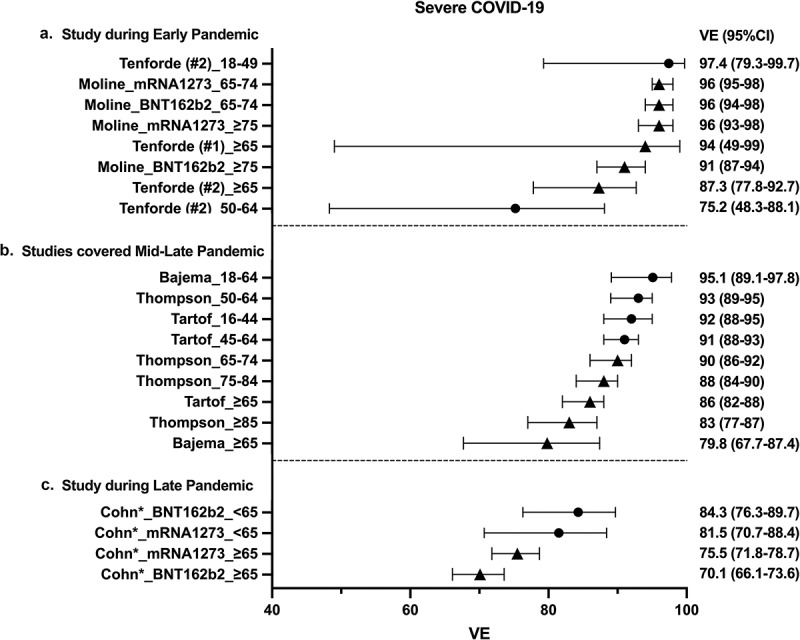
Forest plots of mRNA ve against severe COVID-19.
VE data against severe COVID-19 are shown. BNT162b2 and mRNA1273 mRNA VE were evaluated at least seven days after the second vaccination. Severe COVID-19 was defined as hospital admission with the exception of the *Chon et al study which defined severe COVID-19 as death. Circle symbols represents data for young adults (<65 years of age) and triangle symbols represents data for older adults (≥65 years of age). VE: vaccine effectiveness, CI: confidence interval.
VE among young adults
Two studies examined VE against SARS-CoV-2 infection among young adults (Table 1).8,14 Estimate of VE against SARS-CoV-2 infection was 89% in an early study (paper 1),8 and 73% in a study which evaluated VE during 14 December 2020 and 8 August 2021 (paper 7).14 Five studies examined mRNA VE against severe COVID-19 among young adults (Table 1).11–15 Estimate of VE against COVID-19 hospitalization ranged from 75–97% and 91–95% among an early study (paper 4) and studies from the late phase (papers 5–8), respectively.9,11–14 A late period study (paper 8) evaluated VE against severe COVID-19 defined as death and reported VE for young adults that ranged from 81.5% (for mRNA1273) to 84.3% (for BNT162b2) (Figure 2).15
Discussion
To our knowledge, ours is the first review to report age-disaggregated VE of SARS-CoV-2 mRNA vaccines focusing on durability of VE in older adult. We found considerable declines in VE against SARS-CoV-2 infection in older adults (≥65 years of age) among the studies which captured VE during mid to late pandemic phase to 8 August 2021. Older adults also demonstrated decline in VE against severe COVID-19 during this period. Furthermore, marked declines in VE against severe COVID-19 were reported in a late study among a cohort of veterans ≥65 years of age. The study demonstrated that VE against death dropped to 70% for BNT162b2 and 76% for mRNA1273.15 These data contribute to the evidence base for the recent FDA EUA and CDC recommendation of a booster dose of COVID-19 vaccine in older adults ≥65 years of age, and should be considered in the context of significantly higher risk of severe illness from COVID-19 for the older population.
It is important to focus on VE against severe COVID-19 in older adults. Increasing age is the most attributable risk factor of COVID-19-related death among non-vaccinated population.3 Furthermore, several articles have reported weaker vaccine-induced immune responses in older adults, including lower concentrations of neutralizing antibodies, compared to younger adults.16–20 Although mRNA vaccines were highly effective against severe COVID-19 in older adults after the immunization, the current study suggests that waning VE might lead to severe outcome including death.
We also captured VE of younger adults from eligible studies. We found that the VE against severe COVID-19 maintained >90% in young adults while older adults showed substantial decline resulted in ≤90% of VE in the studies which analyzed VE during the mid pandemic period. Although these data suggest that VE may wane in older adults faster than young adults, we should be cautious when interpreting the current data because the observation periods after vaccination might differ between young and older adults. For example, the SARS-CoV-2 vaccine campaign in Massachusetts prioritized older adults, starting their immunization in 1 February 2021 whereas those <55 years of age began receiving SARS-CoV-2 vaccines in 19 April 2021.21 Because we did not perform a formal meta-analysis within this paper, we do not attempt to conclude whether VE is waning significantly in older adults compared to younger adults. Furthermore, a study included in our review, which analyzed VE between July and October 2021 showed waning VE against death in veterans <65 years to 81.5–84.3%.15 We should bear in mind that the veterans analyzed in the study had a skewed distribution of sex and age. Nonetheless, these results suggest that VE against severe COVID-19 and death is also waning in younger adults.
Waning VE has been more pronounced with the emergence of Omicron, the latest VOC first reported to WHO on 24 November 2021. Thereafter, Omicron rapidly became predominant and demonstrated lower antibody neutralization by vaccine induced immunity.22 Several studies reported that VE against severe COVID-19 induced by Omicron significantly dropped compared to previous strains, including the delta variant. For example, a study from South Africa reported that VE for adults ≥18 years against COVID-19 hospitalization was 23% lower in omicron era compared to the previous period when delta was predominant.18 At the time of this review, limited data were available for VE of older adults against omicron-induced severe COVID-19, an important question that will require ongoing close observation.
There are several other limitations to our review. First, since there was little to no information regarding the dominant SARS-CoV-2 strain among the studies, circulating SARS-CoV-2 strains were not accounted for in our review. mRNA vaccine VE against SARS-CoV-2 infection is decreasing with increasing report of breakthrough infections.4,5,15 This is likely due to both waning immunity occurring months after vaccinations, and an emergence of SARS-CoV-2 variants which become predominant during the study period. Several SARS-CoV-2 variants harboring mutations are associated with decreased mRNA VE against infection and/or severe COVID-19 due to increased transmissibility and immune escape from vaccine induced immune memory.23 Each variant differs with respect to its clinical significance. For example, Omicron has demonstrated increased infectivity and immune escape but to have lower clinical severity than previous strains.22,24,25 Changes in circulating SARS-CoV-2 strains might have affected VE in this review, and multiple variables (eg, time since vaccination and viral strains) make it difficult to generalize data. Second, only U.S.-based studies were included. Our review focused on these studies for several reasons: (1) the initiation and progression of vaccination campaigns varied between countries; (2) some countries applied a modified vaccine regimen (e.g. prolonged interval between 1st and 2nd dose); and (3) the predominant circulating SARS-CoV-2 strain differs between countries. Future reviews should broaden scope to include studies conducted outside the U.S. Third, definition of VE (e.g. 7 vs 14 days after second dose, hospitalization vs death) and study design (e.g. test-negative, cohort registry) varied between the studies. Due to the above limitations, we did not pool VE data nor perform meta-analysis to avoid potential bias. Fourth, we only considered VE estimates for young adults when they appeared in the same articles which included estimates of VE in older adults. This approach enabled us to match study backgrounds between young and older adults, but the studies included in this review might not be representative of the broader population of young adults. Fifth, some studies had an overlap between their observation periods. We defined three calendar periods based on phase of pandemic and emergence of the delta variant. However, some of the papers have a study period that covers more than one period. Finally, only one study analyzed VE exclusively during the late pandemic period. These limitations should be carefully considered when interpreting the results.
In summary, we found that VE against SARS-CoV-2 infection and severe COVID-19 wanes in older adults. These results are consistent with recent policies recommending a booster dose of COVID-19 vaccine in older adults ≥65 years of age. Overall, our vaccine response to the coronavirus pandemic should consider precision vaccinology principles, such as accounting for age as a key biologic variable.
Acknowledgments
We thank our fellow faculty within the Precision Vaccines Program (PVP) for helpful conversations as well as the PVP administrative staff for their support.
Funding Statement
The PVP is supported in part via NIH/NIAID grants and contracts including Adjuvant Discovery [HHSN272201400052C and 75N93019C00044] and Development [HHSN272201800047C] Program Contracts as well as the BCH Department of Pediatrics.
Disclosure statement
OL is a part time employee of the U.S. FDA and a named inventor on patents relating to vaccine adjuvants and human in vitro models that model vaccine action.
References
- 1.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–6. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Marc GP, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–36. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowlkes A, Gaglani M, Groover K, Thiese MS, Tyner H, Ellingson K.. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection among frontline workers before and during B.1.617.2 (Delta) Variant Predominance - Eight U.S. Locations, December 2020-August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1167–69. doi: 10.15585/mmwr.mm7034e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nanduri S, Pilishvili T, Derado G, Soe MM, Dollard P, Wu H, Li Q, Bagchi S, Dubendris H, Link-Gelles R, et al. Effectiveness of Pfizer-BioNtech and moderna vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant - National Healthcare Safety Network, March 1-August 1, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1163–66. doi: 10.15585/mmwr.mm7034e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FDA Authorizes Booster Dose of Pfizer-BioNTech COVID-19 Vaccine for Certain Populations. The U.S. Food and Drug Administration. [Google Scholar]
- 7.COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. [Google Scholar]
- 8.Young-Xu Y, Korves C, Roberts J, Powell EI, Zwain GM, Smith J, Izurieta HS.. Coverage and estimated effectiveness of mRNA COVID-19 vaccines among US veterans. JAMA Netw Open. 2021;4:e2128391. doi: 10.1001/jamanetworkopen.2021.28391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tenforde MW, Olson SM, Self WH, Talbot HK, Lindsell CJ, Steingrub JS, Shapiro NI, Ginde AA, Douin DJ, Prekker ME, et al. Effectiveness of Pfizer-BioNtech and moderna vaccines against COVID-19 among hospitalized adults aged ≥65 years - United States, January-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:674–79. doi: 10.15585/mmwr.mm7018e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moline HL, Whitaker M, Deng L, Rhodes JC, Milucky J, Pham H, Patel K, Anglin O, Reingold A, Chai SJ, et al. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults aged ≥65 years - COVID-NET, 13 States, February-April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1088–93. doi: 10.15585/mmwr.mm7032e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tenforde MW, Patel MM, Ginde AA, Douin DJ, Talbot HK, Casey JD, Mohr NM, Zepeski A, Gaglani M, and McNeal T , et al. Effectiveness of SARS-CoV-2 mRNA vaccines for preventing Covid-19 hospitalizations in the United States. Clin Infect Dis. 2021;ciab687. doi: 10.1093/cid/ciab687. [DOI] [Google Scholar]
- 12.Thompson MG, Stenehjem E, Grannis S, Ball SW, Naleway AL, Ong TC, DeSilva, MB, Natarajan, K, Bozio, CH, Lewis, N, et al. Effectiveness of Covid-19 vaccines in ambulatory and inpatient care settings. N Engl J Med. 2021;385:1355–71. doi: 10.1056/NEJMoa2110362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bajema KL, Dahl RM, Prill MM, Meites E, Rodriguez-Barradas MC, Marconi VC. Effectiveness of COVID-19 mRNA vaccines against COVID-19-associated hospitalization - five veterans affairs medical centers, United States, February 1-August 6, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1294–99. doi: 10.15585/mmwr.mm7037e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, Frankland TB, Ogun OA, Zamparo JM, Gray S, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet (London, England). 2021;398:1407–16. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, and Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science (New York, NY). 2021;375:331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384:80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bates TA, Leier HC, Lyski ZL, Goodman JR, Curlin ME, Messer WB, Tafesse FG. Age-Dependent neutralization of SARS-CoV-2 and P.1 variant by vaccine immune serum samples. Jama. 2021;326(9):868–69. doi: 10.1001/jama.2021.11656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collier DA, Ferreira I, Kotagiri P, Datir RP, Lim EY, Touizer E, Meng B, Abdullahi A, Elmer A, Kingston N, et al. Age-Related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596:417–22. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, Doolman R, Asraf K, Mendelson E, Ziv A, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller L, Andrée M, Moskorz W, Drexler I, Walotka L, Grothmann R, Ptok J, Hillebrandt J, Ritchie A, and Rabl D, et al. Age-Dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis. 2021;73:2065–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massachusetts’ COVID-19 vaccination phases. Commonwealth of Massachusetts. [Google Scholar]
- 22.Garcia-Beltran WF, St Denis KJ, Hoelzemer A, Lam EC, Nitido AD, Sheehan ML, et al. mRNA-Based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185(3):457–66.e4. doi: 10.1016/j.cell.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao K, Tzou PL, Nouhin J, Gupta RK, de Oliveira T, Kosakovsky Pond SL, Berrios C, Ofoman O, Chang CC, and Hauser BM, et al. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat Rev Genet. 2012;22:757–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Accorsi EK, Britton A, Fleming-Dutra KE, Smith ZR, Shang N, Derado G, Miller J, Schrag SJ, Verani JR. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and Delta variants. Jama. 2022;327(7):639. doi: 10.1001/jama.2022.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolter N, Jassat W, Walaza S, Welch R, Moultrie H, Groome M, Amoako DG, Everatt J, Bhiman JN, Scheepers C, et al. Early assessment of the clinical severity of the SARS-CoV-2 Omicron variant in South Africa: a data linkage study. Lancet (London, England). 2022;399:437–46. doi: 10.1016/S0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


