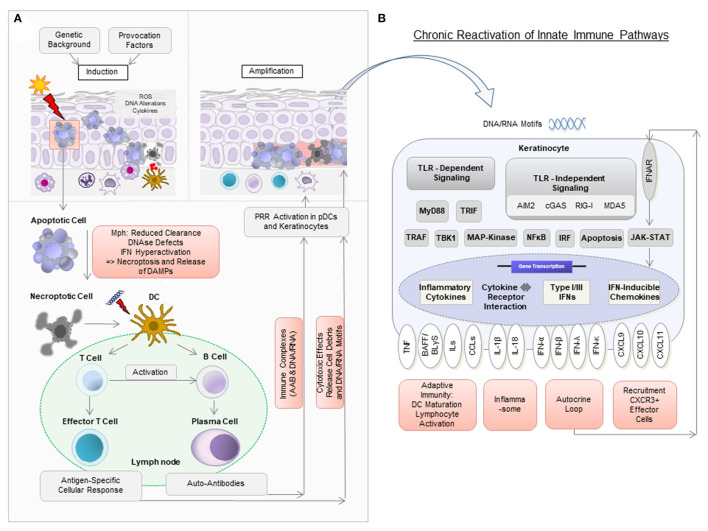
Figure 1.
Model of pathogenic mechanisms in cutaneous lupus erythematosus (CLE). (A) In a person with a genetic background predisposing to CLE, the exposure to provocation factors such as UV light can induce cellular stress [reactive oxygen species (ROS), DNA alterations, cytokine secretion], apoptosis, and the release of DNA components in so-called “apoptotic blebs” in keratinocytes. Normally, these “blebs” are rapidly degraded and apoptotic cells are removed by macrophages (Mph). In CLE, delayed degradation and clearance leads to secondary, more pro-inflammatory, forms of cell death such as necroptosis, which results in the release of cell debris. Dendritic cells (DC) recognize this debris as potential autoantigens and migrate to nearby lymph nodes to present it to T and B cells. Upon activation, naïve B cells develop into plasma cells to produce autoantibodies (AAB). AAB form immune complexes with nucleic acids and can induce type I IFN production in plasmacytoid dendritic cells (pDCs). T cells mature into CD4+ and CD8+ T cells, with the latter exerting cytotoxic effects against keratinocytes. (B) Nucleic acids (DNA and RNA motifs) released from dying cells can be recognized by pattern recognition receptors (PRR) as so-called damage-associated molecular patterns (DAMPs), leading to activation of both Toll-like receptor (TLR)-dependent and TLR-independent inflammatory signaling cascades. In CLE, this leads to increased expression of several cytokines, particularly type I IFN. Type I IFN is known to bind to IFN-α/β receptors on keratinocytes in an autocrine loop and mediates increased expression of proinflammatory chemokines such as CXCL10 via the JAK-STAT pathway. This leads to the recruitment of CXCR3+ cells, which induce keratinocyte cell death, release of cytokines and a chronic reactivation of innate immune pathways.