Figure 1.
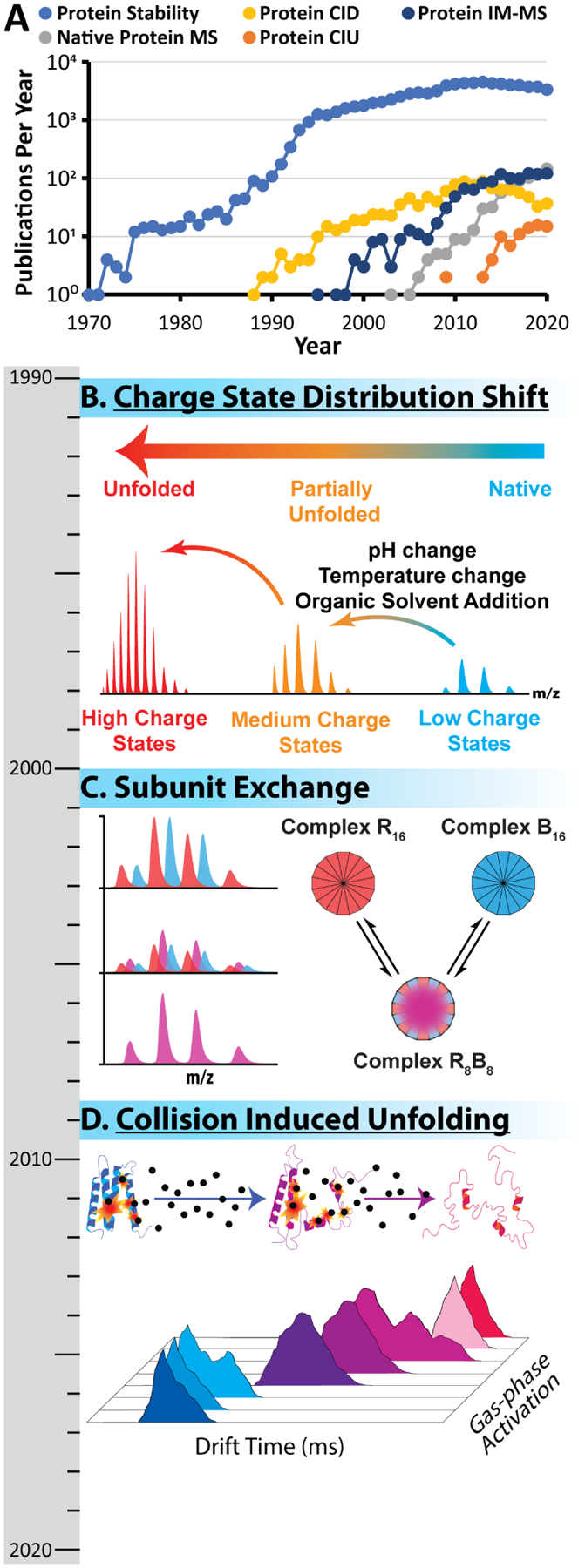
A) The total number of publications in the pubmed.gov database for the search terms indicated in legend. B) The majority of early protein stability measurements conducted by MS (pre-2000) relied on the observation of CSD shifts. The blue CSD represents a “native” protein possessing lower charge states. Upon denaturation, the protein unfolds presenting a CSD centered at higher charge (lower m/z), occupying partially unfolded structures (orange CSD) enroute to a fully unfolded population (red CSD). C) Native MS emerged in the early 2000s, and one of the initial biological systems studied by this approach were α-crystallin proteins. MS is able to track the exchange between co-incubated homo-oligomers, which eventually leads to the formation of hetero-oligomers. D) Building upon these initial measurements, CIU employs gas phase activation in conjunction with IM, to observe the gas phase change in structure due to collisional heating. This approach is growing rapidly and is being applied to a broad variety of biological systems.
