Figure 5. CIU applications for biotherapeutic mAbs.
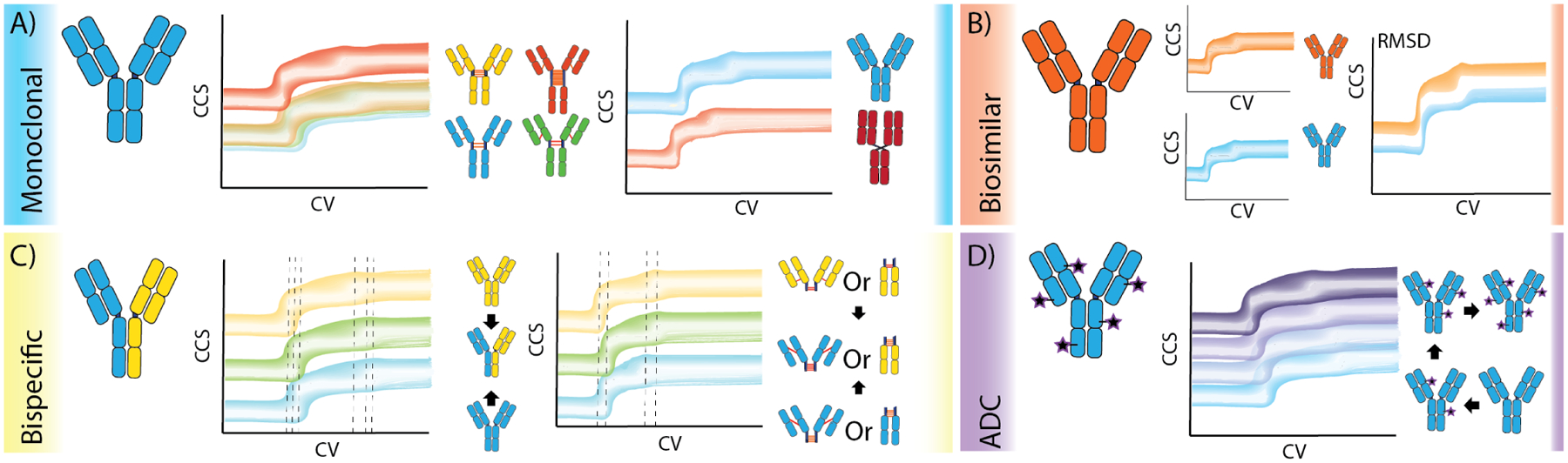
A) Differentiation of monoclonal IgG subclasses by disulfide bonding patterns and difference in CIU unfolding due to domain exchange. B) Biosimilar antibodies have qualitatively similar fingerprints, but contemporary CIU analyses are able to quantitate subtle differences in stability. C) Bispecific antibodies present CIU characteristics centered between the precursor structures. D) Shifts in CCS and stability can be quantified as a function of increasing drug load in ADC biotherapeutics.
