Figure 7.
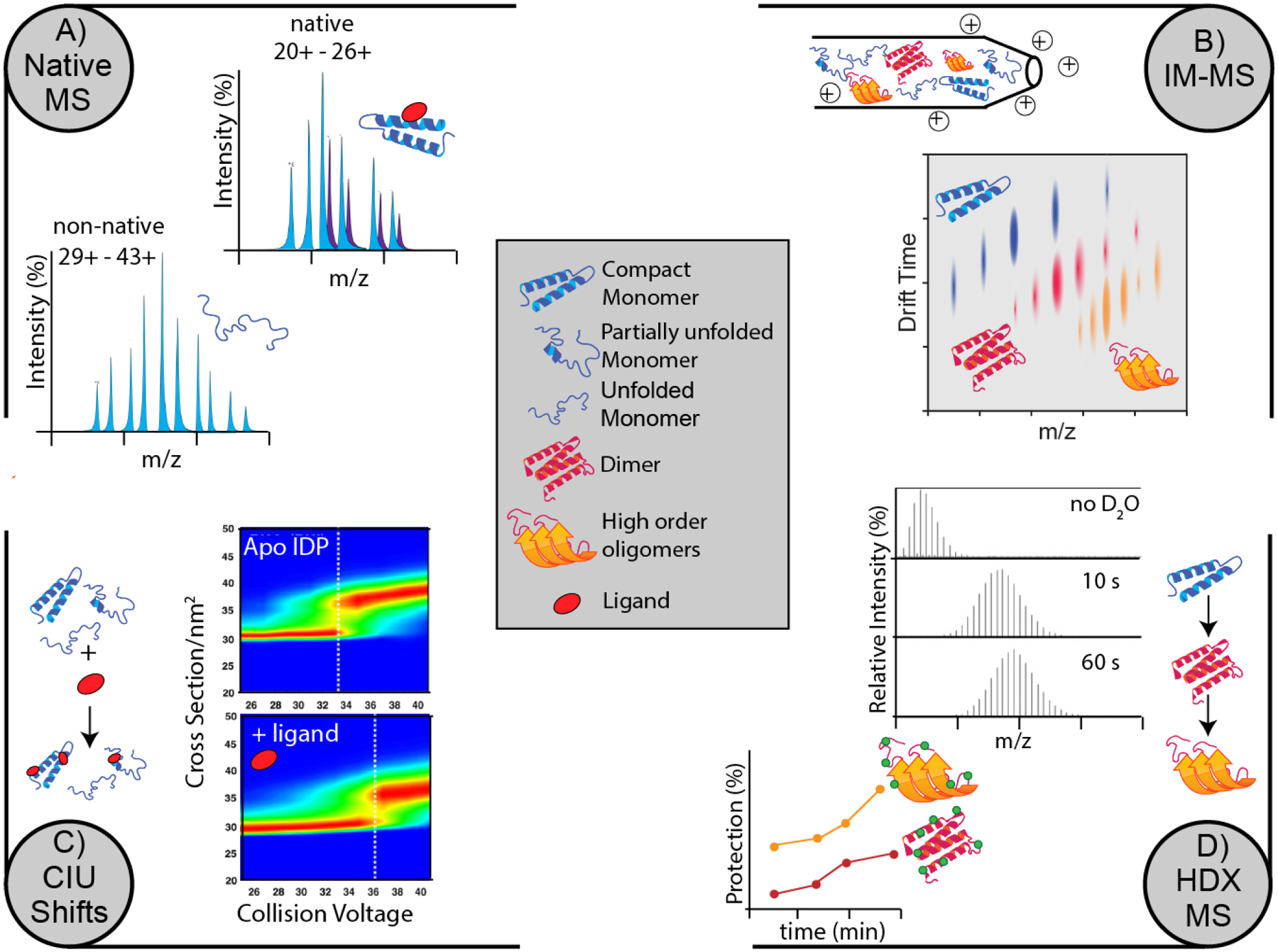
Overview of select MS techniques that allow direct stability measurements with challenging amyloidogenic proteins A) Native MS usually produces a narrow range of charge states compare to non-native MS. Native MS can retain the native structures of amyloidogenic proteins and even the non-covalent complexes formed through ligand or protein binding through gentle ionization parameters. B) nESI needle is filled with a mixture of different oligomers of an amyloidogenic protein. IM-MS is able to separate the complex population of oligomers in drift time space based on their size, charge and shape. C) CIU shifts otherwise known as CIU50 values can be obtained through a series of IM-MS experiments at increasing collision energy. If a ligand binding event caused an amyloid protein to increase in stability, CIU50 values will reflect this increase. D) HDX-MS can capture localized information, in as little as few milliseconds or as long as days, allowing us to take snapshots of an amyloid aggregation process
