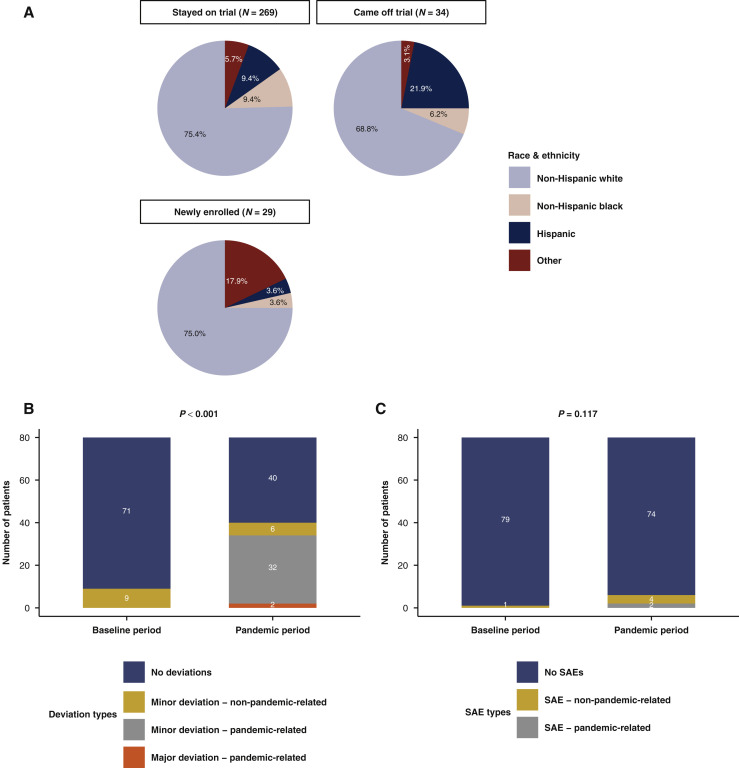
Figure 6.
(A) Pie charts representing the breakdown of race and ethnicity by clinical trial participation group in the manually curated cohort (n = 332). (B) Stacked bar plot representing clinical trial deviations during the baseline and pandemic periods (P value from Fisher’s exact test for deviation versus no deviation reported) in the manually curated cohort (n = 332). (C) Stacked bar plot representing clinical trial serious adverse events during the baseline and pandemic periods (P value from Fisher’s exact test for deviation versus no deviation reported) in the manually curated cohort (n = 332). The baseline period was defined as the pre-pandemic period from December 2019 to March 2020. The pandemic period was defined as the first peak of the pandemic of the Northeastern United States from March to June 2020.
SAE, serious adverse event.