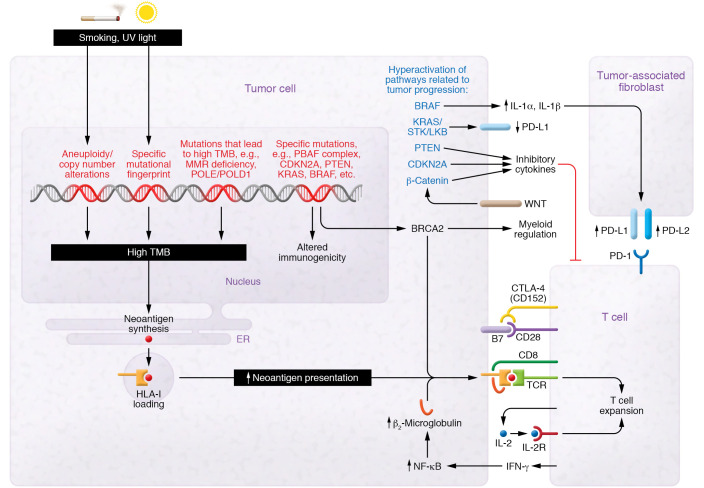
Figure 1. Genetic alterations and immunologic consequences.
Certain mutagens, such as UV light and carcinogens in cigarette smoke, can lead to formation of mutations and aneuploidy, which can cause a high tumor mutational burden (TMB). Other specific alterations can lead to microsatellite instability or POLE/POLD1 mutation, which results in hypermutation. This can result in transcription and translation of tumor neoantigens. These are presented on HLA-I molecules. HLA-I is required to present tumor neoantigens to cytotoxic T cells. Certain mutations, such as KRAS/STK/LKB in lung cancer, have been associated with decreased PD-L1 expression on tumor cells. Other mutations in genes such as BRAF, CDKN2A, and PTEN as well as aberrant activation of the WNT/β-catenin pathway have been implicated in increasing the release of inhibitory cytokines in the tumor microenvironment that act on tumor-infiltrating T lymphocytes or tumor-associated fibroblasts. T cells recognize antigens presented on HLA-I as “non-self” antigens; costimulatory signals are needed for Th cell activation. Costimulatory signals involve the binding of B7 on tumor or antigen-presenting cells to CD28 on T cells. CTLA-4 (CD152) competes with CD28 for the binding of B7, thus inhibiting the necessary costimulatory signal needed. Owing to space constraints, this diagram is not comprehensive.