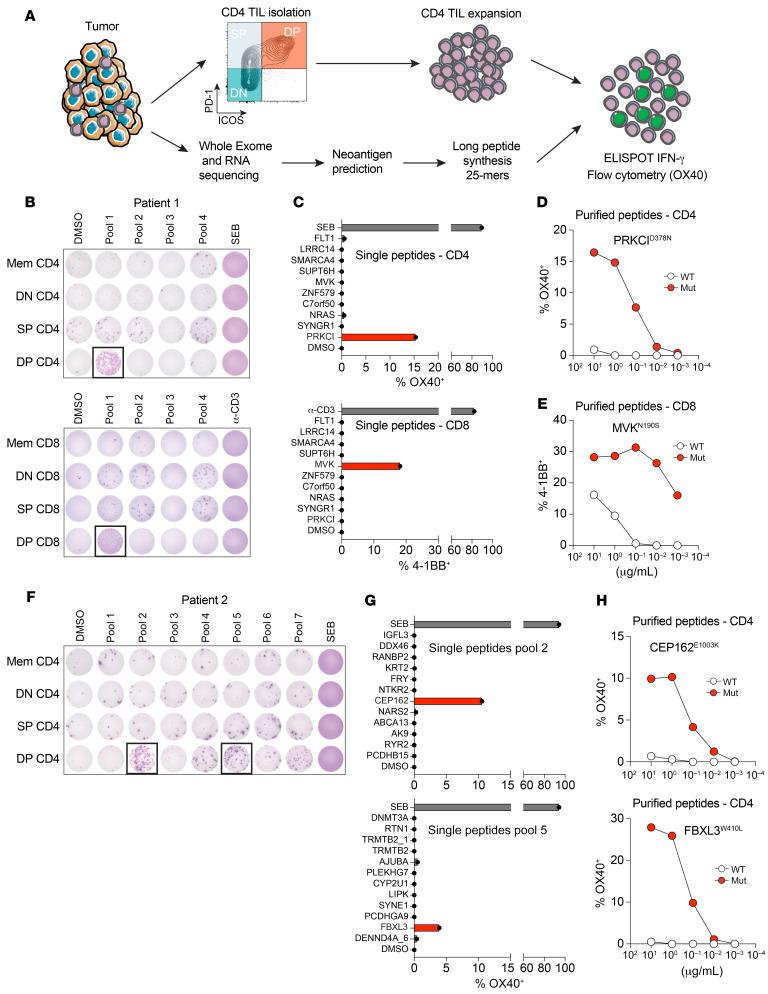
Figure 8. DP CD4+ Th TILs recognize tumor-specific neoantigens.
(A) Schematic representation of the methodology used to identify neoantigen-reactive CD4+ Th cells. (B) In vitro–expanded CD4+ and CD8+ T cell subsets (DN, SP, and DP) from patient 1 were cocultured with autologous B cells pulsed with DMSO or the indicated peptide pools. T cell reactivity was measured by IFN-γ ELISPOT assay. (C) Reactivity of DP CD4+ and DP CD8+ TILs to B cells pulsed with individual 25 mer peptides from pool 1. Flow cytometric analysis of OX40 or 4-1BB upregulation on CD4+ or CD8+ T cells, respectively is shown. The mutations recognized are highlighted in red. (D) DP CD4+ Th TILs were cocultured with autologous B cells pulsed with decreasing concentrations of WT or mutated (Mut) PRKCID378N 25 mer peptides. Reactivity was measured by flow cytometric analysis of OX40 upregulation on CD4 cells. (E) DP CD8+ TILs were cocultured with autologous B cells pulsed with decreasing concentrations of WT or mutated 8 mer MVKN190S peptides. Reactivity was measured by flow cytometric analysis of 4-1BB upregulation on CD8+ T cells. (F) In vitro–expanded CD4+ T cell subsets (DN, SP, and DP) from patient 2 were cocultured with autologous B cells pulsed with DMSO or the indicated peptide pools. T cell reactivity was measured by IFN-γ ELISPOT assay. (G) Reactivity of DP CD4+ Th TILs to B cells pulsed with individual 25 mer peptides from peptide pools 2 and 5. Flow cytometric analysis of OX40 upregulation on CD4+ cells is shown. The mutations recognized are highlighted in red. (H) DP CD4+ Th TILs were cocultured with autologous B cells pulsed with decreasing concentrations of WT or mutated CEP162E1003K or FBXL3W410L 25 mer peptides. Reactivity was measured by flow cytometric analysis of OX40 upregulation on CD4+ cells.