FIGURE 1. Intracranial and overall response rates to ALK tyrosine kinase inhibitor therapy in ALK-positive lung cancers.
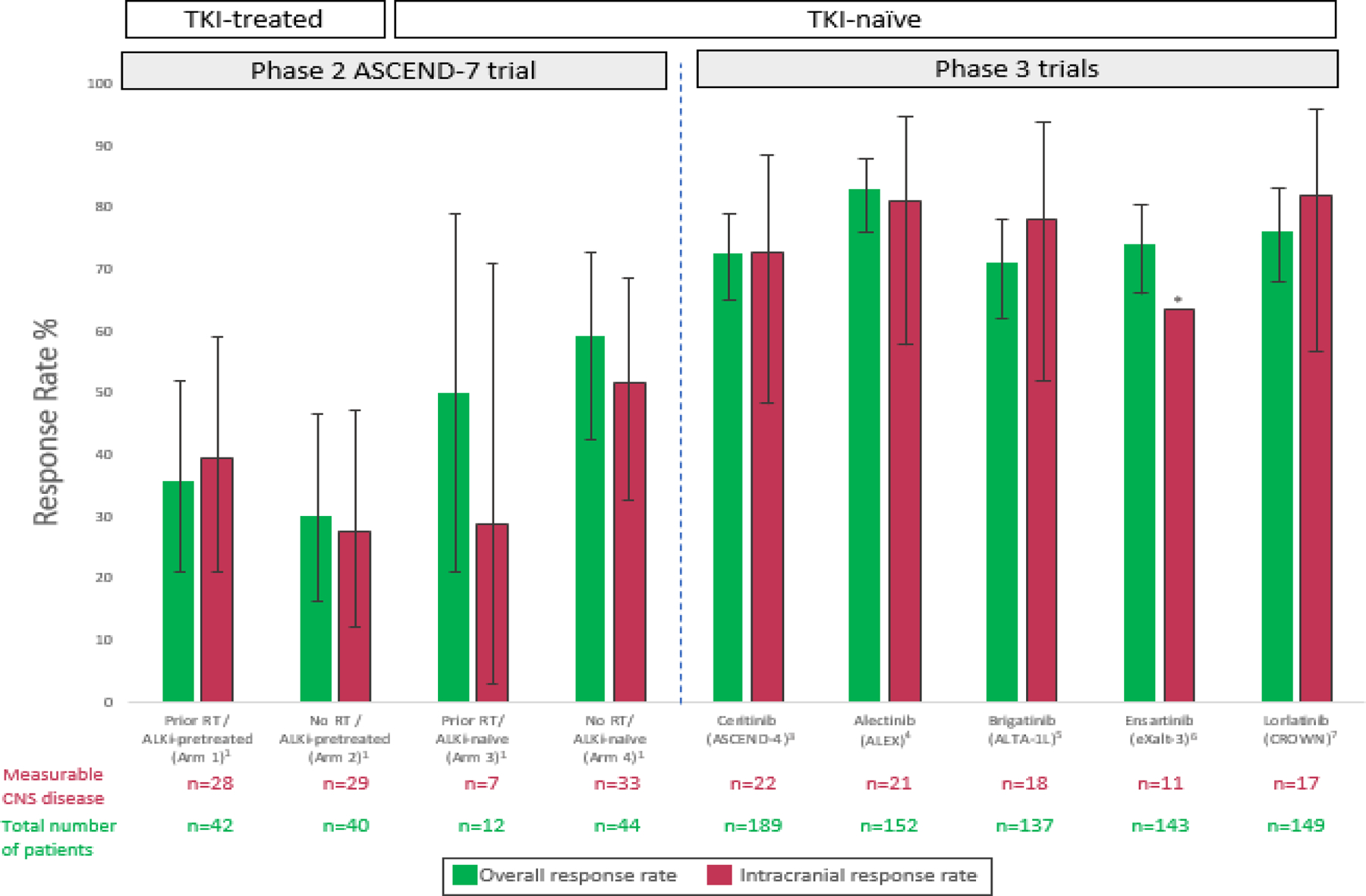
The intracranial and overall response rates to ceritinib and select other second and third-generation ALK tyrosine kinase inhibitors are shown. Notably the cohort of patients enrolled in the ASCEND-4 study of ceritinib included some with neurologically stable CNS disease, but eligibility was more lenient in ASCEND-7, which was designed as a dedicated CNS study. Error bars indicate 95% confidence intervals (CI) where available. The * symbol indicates that information on the 95% CI was not available.
