Abstract
Microbial symbionts in the gut are increasingly recognized as having important effects on health and disease, but have only recently begun to be linked to diseases of the eye. We review current research on the intestinal microbiota’s relationship to ocular disease, focusing on autoimmune uveitis, diabetic retinopathy, age-related macular degeneration and primary open angle glaucoma. We discuss findings and limitations of this exciting new area of ophthalmology research and explore possible future disease-modifying treatments.
Keywords: gut microbiome, glaucoma, age-related macular degeneration, diabetic retinopathy, autoimmune uveitis
Introduction
The human gut microbiota comprises an estimated 4×1013 bacteria[1] and other microorganisms[2] which play important reciprocally regulated roles in host digestion and absorption,[3] immune function,[4,5] vitamin and amino acid synthesis,[6] and drug metabolism.[7,8] Dysbiosis of the gut has well-supported ties to a host of human diseases, including cardiovascular disease,[9,10] metabolic syndrome and diabetes,[11,12] gastrointestinal and systemic autoimmune disease,[13] and cancer, as well as chronic and inflammatory diseases of the eye.[14] Until recently, the effects of microbiota on ophthalmic disease have been less studied than gastrointestinal and systemic diseases. While there is ample research on the relationship between microbiota and systemic conditions (for example, high blood pressure and diabetes) which affect the incidence and progression of eye disease, clinical and basic research on ocular disease specifically has accelerated only in the last few years. Recent evidence aimed at elucidating the relationship between the human eye and gut microbiota supports the presence of a gut-eye axis, i.e. effects of gut microorganism-derived mediators on structures within the eye (Fig. 1).[15,16] In support of the gut-eye axis, many studies have uncovered an association between alterations in gut microbial composition and ocular disease, as well as possible therapeutic effects of manipulating the microbiome with dietary interventions and antibiotic treatment. This review will focus on gut microbiota and intraocular diseases; we will review current research on association of gut microbiota with autoimmune uveitis, diabetic retinopathy, age-related macular degeneration (AMD), and primary open-angle glaucoma (POAG).
Fig. 1.
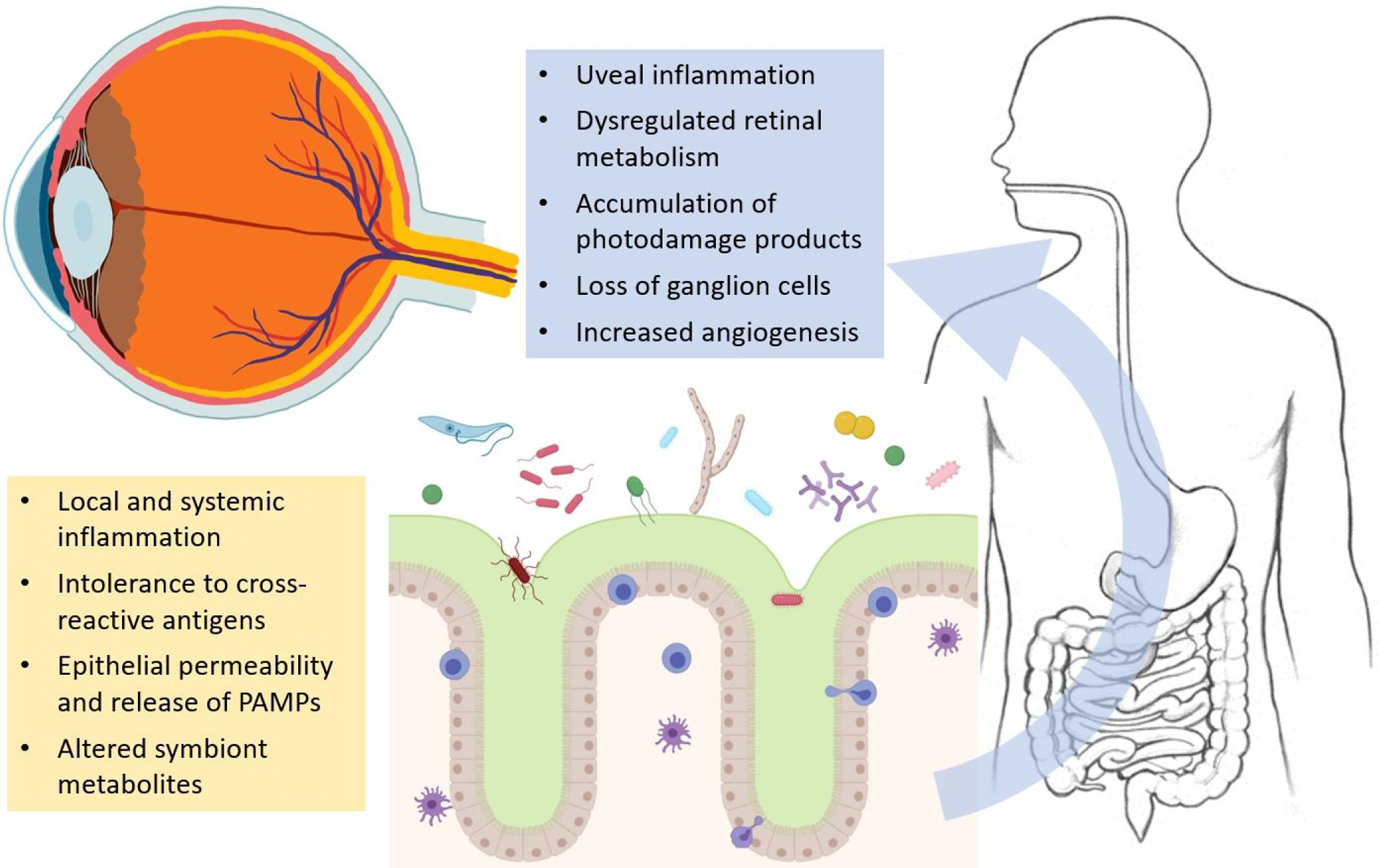
Downstream direct and indirect effects of gut dysbiosis on the eye. Maladaptive changes in the microbial communities overlying oral/intestinal mucosa may be induced by diet, environmental exposures, illness or drugs, causing diverse effects on eye physiology.
Autoimmune uveitis
Uveitis is a disease characterized by inflammation of the uvea, a structure in the eye composed of the choroid, iris, and ciliary body, as well as the neural retina; clinical manifestations vary with the involved anatomy but include redness, pain, photophobia, and loss of visual acuity. Uveitis affects 115.3 per 100,000 people in the United States, with a similar lifetime incidence worldwide, and is reportedly more common in females than in males.[17,18] Inflammation in uveitis can be triggered through either non-infectious or infectious mechanisms, though this article will focus on non-infectious or autoimmune uveitis due to its well-studied relationship to gut microbial dysbiosis. Autoimmune uveitis may be isolated or associated with a wide range of systemic inflammatory conditions; it is a clinically and mechanistically heterogeneous group of diseases. We will discuss the association between gut microbial composition and uveitis and then review the hypothetical mechanisms by which gut dysbiosis may contribute to intraocular inflammation..
In studies on human fecal samples, gut microbial diversity was decreased in the uveitis condition; one study found differences in within-group (alpha) diversity[19] while another found differences in between-group (beta) diversity.[20] Principal component analysis of 16S rRNA sequences of gut microbes in uveitis patients and healthy controls found significantly divergent bacterial communities. Closer analysis of microbial composition found decreased abundance of the genera Ruminococcus and Oscillospora in uveitis patients,[20] while another study did not find a significant difference in gut microbiota composition between uveitis patients and controls.[19] In one study, the genera Faecalibacterium, Bacteroides, Clostridium, and Lachnospira, which are known for their anti-inflammatory properties, were found to be reduced in uveitis patients and Prevotella, a pro-inflammatory genus, to be enriched.[20] These studies were unable to ascertain if observed changes in gut microbial composition were responsible for or a result of disease. Interestingly, gas chromatography-mass spectrometry analysis of fecal samples from anterior acute uveitis patients yielded a significant difference in fecal metabolic phenotype compared to healthy controls.[19] These results suggest that even if changes in microbial composition are undetectable or inconsistent, there could be functional differences in the guts of uveitis patients contributing to disease.
The results of these studies, as well as an extensive literature on associations between gut microbiota and systemic autoimmune diseases linked to uveitis,[13] raise the question of the mechanism by which gut bacteria may affect the eye. There are four proposed mechanisms implicating gut microbial dysbiosis in the pathogenesis of autoimmune uveitis. These mechanisms are microbial metabolites, destruction of the intestinal barrier, imbalance of intestinal immune homeostasis, and antigenic mimicry.[21] In the first hypothesized mechanism, microbes in the gut are capable of producing a number of metabolites, including butyric acid and short-chain fatty acids, which have protective properties in inflammation. Alterations in the microbial composition of the gut can decrease levels of beneficial microbial metabolites, exacerbating inflammation in uveitis. In the second mechanism, a weakened intestinal barrier due to dysbiosis can lead to leakage of pathogenic products of microbes into the systemic circulation. These products such as lipopolysaccharides (LPS) could land in the uvea and elicit an immune response, leading to uveitis.[22] In the third mechanism, an imbalance of T helper 17 (Th17) and T regulatory (Treg) cells results in overproduction of IL-17, stimulating an inflammatory pathway and promoting uveitis.[23,24] The last mechanism involves antigenic mimicry whereby T cells capable of recognizing self-antigens in the uvea are activated in the gut by microbial peptides. One study found that T cells activated by an experimental autoimmune mouse model’s intestinal extracts induced autoimmune uveitis in naïve wild-type mice, supporting antigenic mimicry as an inciting role in autoimmune uveitis[25]. It is likely that more than one of these hypothesized mechanisms are at play in the pathogenesis of autoimmune uveitis as they are not mutually exclusive and reflect a complex interplay of immune cells and gut microbial dysbiosis.
Diabetic retinopathy
Diabetic retinopathy (DR) is one of the cardinal manifestations of microvascular compromise in diabetes mellitus. DR is divided into non-proliferative DR, proliferative DR, and diabetic macular edema. Retinal changes associated with uncontrolled diabetes are typically asymptomatic for years until late stages, when visual compromise may progress rapidly due to vitreous hemorrhage, tractional detachment, secondary glaucoma, or macular edema. For this reason and because of the high prevalence of diabetes, DR is the leading cause of blindness among working adults in developed countries, and is associated with significant healthcare costs.[26–28]. Duration and severity of hyperglycemia as well as high blood pressure are the primary risk factors for development and progression of DR, but patient-specific factors also exist—some people never develop DR despite years of hyperglycemia. Many studies have demonstrated effects of gut microbiota on diabetes,[12] but relatively few have investigated effects specifically on diabetic retinopathy. In this section, we will discuss experimental evidence investigating the relationship between the gut microbiota and DR.
Several studies have investigated the differences in gut microbial composition between diabetic patients with and without DR; the first, using traditional selective culture media techniques, found no significant differences,[29] while the second, using 16S rRNA gene sequencing from 25 patients with diabetes, 28 patients with DR and 30 age- and sex-matched healthy controls,[30] found differences at the genus level, with DR patients’ stool having reduced abundance of both anti-inflammatory and pathogenic genera. More than half of patients in the diabetes cohort of this study had a new (<4 month) diagnosis, whereas the DR patients all had years-long history of diabetes. Treatment with metformin, for example, has been shown to have predictable effects on the gut microbiome that may form part of its mechanism of action.[31] In an effort to determine if DR-associated microbiota changes exist independently of those induced by years of diabetes or its treatment, Khan et al. performed 16S rRNA sequencing on fecal swabs from 37 patients with sight-threatening diabetic retinopathy and 21 matched controls, all with greater than 10 years’ history of diabetes; they found no significant differences in abundance of measured taxa, though the ratio of Bacteroidetes to Firmicutes was found to be significantly different between patients and controls. In a similar vein, Huang et al. evaluated 25 patients with DR, 25 patients with T2DM without DR matched for duration of disease, and 25 healthy controls.[32] Significant differences were found between healthy controls and the DM and DR groups, but few differences were found between DM and DR groups. More research is needed to validate these results and determine if gut microbiota affect DR independent of DM status.
In animal models, several studies have discovered a relationship between a favorable gut microbial environment and improved DR outcomes in diabetic rodent models.[33,34] Intermittent fasting (IF) was found to both induce a shift from bacterial species of Bacteroidetes to Firmicutes in the gut, to decrease the activation of retinal microglia and infiltration of peripheral immune cells into the retina, and to improve overall survival in a type 2 diabetic mouse model.[33] Species belonging to Firmicutes can metabolize primary bile acids to secondary bile acids such as tauroursodeoxycholic acid (TUDCA), a compound found to have protective properties in rat retinal neurons.[34,35] The authors hypothesized that an increased proportion of Firmicutes in the gut could improve DR outcomes through the protective effects of TUDCA, a byproduct of Firmicutes bile acid metabolism. The mechanism by which TUDCA improves DR outcomes was explored in experiments on rat retinas exposed to a diabetic condition.[34–36] TUDCA was found to decrease expression of immune mediators and angiogenic factors such as nitric oxide synthase, ICAM-1, NF-κB p65, and VEGF in diabetic mouse models. In cultured rat retinal neurons exposed to elevated glucose concentrations, TUDCA decreased cell death by attenuating the release of apoptosis-inducing factor (AIF) in mitochondria and reducing oxidative damage.[34] TGR5, a receptor of TUDCA, has been shown to play a role in DR pathology in mouse models.[37] These results suggest that TUDCA may play a number of roles in preventing DR progression such as alleviating inflammation and preventing retinal cell death in DR.
Overall, these studies also propose that dietary modifications such as IF and administration of TUDCA could be used as treatments for DR and other retinal diseases. IF was found to shift the gut microbial community towards larger proportions of Firmicutes, thus increasing bile acid metabolism and TUDCA production.[33] Future research points towards elucidating a more specific gut microbial profile associated with DR and tailoring treatments of retinal diseases involving TUDCA.[38]
Age-related macular degeneration
Age-related macular degeneration (AMD), the leading cause of adult blindness in high-income countries, is a multifactorial disease with incompletely understood pathogenesis, but epidemiologic studies have implicated genetic differences, innate immunity,[39,40] inflammatory markers,[41] diet,[42,43] and specific vitamin[44] intake in AMD incidence and progression. Given that microbiota shape both host immune response and metabolism,[45,46] and that diet shapes gut microbial communities,[47] recently researchers have sought to find effects of microbiota on AMD. Two case-control studies from the same group found that the feces of AMD patients were enriched in bacterial taxa associated with high-fat diet and inflammation, such as Anaerotruncus, and reduced in Bacteroides spp., which are associated with protection from autoimmune disease and fermentation of indigestible carbohydrates.[48,49] The authors found associations between the complement system and gut microbiome changes in C3−/− mice--a genetic background that negatively affects aged retinas—including increases in Firmicutes-to-Bacteroidetes ratio and the abundance of order Clostridiales. In humans, similar gut microbiome changes were correlated with single nucleotide polymorphisms (SNPs) in the complement factor H gene, suggesting a relationship between complement deficiency, specific gut microbiome changes, and AMD. Metabolic pathway inferences suggested that gut bacteria of AMD patients have reduced fatty acid elongation and increased L-alanine fermentation, glutamate degradation and arginine biosynthesis, which may plausibly affect retinal health.[50,51] Corroborating these studies, Andriessen and coworkers found that wild-type mice fed a high-fat diet have both greater choroidal neovascularization in response to experimental laser injury and an increased ratio of gut bacteria in phylum Firmicutes at the expense of Bacteroidetes, as well as increased gut dye permeability and measures of systemic and choroidal inflammation.[52] Normalization of gut bacterial phyla via fecal transplant restored normal-diet levels of laser-induced choroidal neovascularization, regardless of diet, indicating that gut microbiota are a necessary intermediary for diet-induced increases in choroidal angiogenesis. Rowan and co-workers found that mice fed a high-glycemic index diet develop features similar to dry AMD, including retinal pigmented epithelium depigmentation and atrophy, as well as changes in gut bacteria. As in prior studies, both the high-glycemic-index diet and worse retinal pathology were associated with increased abundance of phylum Firmicutes (including Clostridia) and reduced abundance of phylum Bacteroidetes; the authors identified reduced bacteria-derived serotonin as a diet-independent factor in retinal damage.[53] It is challenging to generalize to human disease from animal models of AMD since only the primate eye has a macula,[54] but these results suggest that a shift in gut microbes may be a factor in AMD pathogenesis. Mechanisms may include increased systemic inflammation by permeation of antigens through a compromised intestinal mucosal barrier, bacterial metabolism of lipids or neurotransmitters, or bioavailability of dietary vitamins or micronutrients. This work merits further validation in human patients with AMD.
Finally, a role for oral dysbiosis in AMD has also been investigated in two case-control studies. In a cohort of 311 mixed AMD patients and 421 healthy controls in Singapore, Ho and co-workers found that pharyngeal microbiota were similar in overall structure and diversity between AMD and control patients, with differences in the abundance of specific genera.[55] Prevotella spp. was found to be relatively reduced, and Gemella and Streptococcus spp. relatively enriched, in AMD; this difference was found to be larger and Leptotrichia spp. also reduced when stratifying for late AMD. Prevotella spp. being less common in AMD is surprising, since members of genus cause periodontal disease[56] and are associated with autoimmune arthritis.[57] In a small case-control study of oral and nasal microbiota, Rullo and co-workers found large differences in many taxonomic units of bacteria, including some linked to atherosclerosis, and recapitulated the prior study’s finding of enrichment of Gemella spp. in AMD.[58] Both studies suffered from a lack of baseline matching between the AMD and control cohorts. More work needs to be done to establish a role for oral or nasal microbiota in AMD.
In summary, multiple studies in mice and humans have identified changes in gut bacteria, specifically an increased fecal Firmicutes:Bacteroidetes ratio, as a possible factor in both neovascular and dry AMD. More work needs to be done to validate these findings in wider groups of patients, and to elucidate whether these changes are a diet-dependent. Studies of oral microbiota in AMD have not produced convincing evidence of an association.
Glaucoma
Glaucoma is a group of neurodegenerative diseases of the optic nerve head associated with increased intraocular pressure (IOP); together they are the world’s leading cause of irreversible blindness. Many theories have been promulgated to explain the observed patterns of neurodegeneration in patients with glaucoma and their relationship with elevated IOP, but none have prevailed. Since autoantibodies to retinal[59] and optic nerve antigens[60] were discovered in glaucoma patients in the 1990’s, autoimmunity has been hypothesized to play various roles (including protective ones) in glaucomatous neurodegeneration. In addition to circulating antibodies, abnormal T-cell repertoires have been identified in glaucoma patients.[61] Recently, work on the effects of microbiota on the intraocular pathology of glaucoma has accelerated, often focused on autoimmune or inflammatory aspects. Some of this work on glaucoma has been reviewed previously,[14,62–64] and may be divided into studies of oral microbiota and studies of gut microbiota, including gastric H. pylori colonization and gut microbiota-mediated autoimmunity to heat shock proteins. The one study that does not fit into these categories, interestingly, performed stool 16S RNA sequencing and serum GC-MS metabolomics to explore differences in microbiota-mediated metabolism in 30 POAG and 30 matched controls.[65] The authors found that stool samples of POAG patients were relatively enriched in E. coli and Prevotellaceae and relatively reduced in Bacteroides plebeius and Megamonas spp and linked these taxa to specific metabolites that may play a role in glaucoma pathogenesis.
Based on a well-tread hypothesis that innate or adaptive immune response to the oral biofilm may mediate neurodegenerative changes,[66] as well as evidence of a role for both complement cascade[67] and the TLR4 receptor[68,69] in glaucoma, several authors have pursued a link between oral microbiota and glaucoma. Promising initial results[69] of almost 2-fold differences in oral bacterial counts in POAG patients vs matched controls were not replicated in a prospective follow-up study.[70] Prospective data from the Health Professionals Follow-up Study[71] and a large retrospective cohort study in Taiwan[72] found inconsistent connections between oral health and risk of glaucoma, with the former study finding that only recent tooth loss alone or tooth loss and periodontal disease was associated with POAG, while the second study found that only periodontal disease was associated with POAG. The link between oral health and glaucoma remains unclear.
Glaucoma was first associated with gut microbiota in 2000, when histologically confirmed gastric H. pylori infection was found in 88% of glaucoma patients vs 47% of anemic controls by Kountouras and co-workers.[73] Subsequent studies using serology and/or [13C]urea breath testing found mixed results,[74] but the most recent meta-analyses overall found evidence of an association between active H. pylori infection and POAG.[75,76] H. pylori infection has been hypothesized to worsen glaucoma via systemic inflammation and increased vasoactive and reactive oxygen species[68] or antibody-dependent responses to cross-reactive ocular antigens.[77] One study even found H. pylori coccoid forms in trabecular and iris specimens from POAG patients,[78] a surprising but likely artifactual finding given that H. pylori is an obligate colonizer of gastric mucosa.[79] Successful eradiation of H. pylori infection in small trials of POAG patients has been found to improve both IOP[80] and visual fields,[81] but eradication in patients with peptic ulcer disease without glaucoma, however, did not change the risk of developing POAG.[82] There remains insufficient evidence to determine if a causative relationship exists, or if the observed association arises from shared susceptibility; subsequent studies on gut microbiota in POAG have taken the observed association with H. pylori infection as an indication that intestinal dysbiosis is a risk factor for both diseases.[64]
The third and final line of investigation into microbiota and glaucoma concerns gut microbiota-mediated immune responses to heat shock proteins (HSPs), which are a large family of molecular chaperones that play diverse roles in signaling and stress response. Autoantibodies to small HSPs were identified in the serum of patients with POAG in 1998,[83] quickly followed by attempts to determine their role in glaucoma pathogenesis.[84,85] HSPs are both immunogenic and highly conserved, and loss of tolerance to commensal bacterial HSP homologs has been proposed to contribute to many autoimmune and neurodegenerative diseases.[86] In 2018, Chen and co-workers provided compelling evidence for this model, showing that transient IOP elevation in mice causes HSP-specific T-cells to infiltrate the retina and contribute to RGC and axon loss, and that this process is attenuated in a low-diversity gnotobiotic mouse model and abolished in germ-free mice.[87] They then generated a germ-free version of the DBA/2J mouse model of hereditary glaucoma and found that while those animals developed the expected elevation in IOP, they had no RGC and minimal axon loss by 12 months of age. Furthermore, the authors identified HSP-reactive T-cells in the peripheral blood of glaucomatous patients but not healthy patients or those with other diseases. The authors integrated these findings into a two-hit model of glaucoma pathogenesis: first, exposure to commensal bacteria in some way primes a T-cell response against self-antigens; second, elevated IOP (or another insult) allows T-cell infiltration into the retina and stimulates retinal cells to express stress factors that become the target of a sustained immune response that drives neurodegeneration and vision loss. These striking results raise several questions. First, Rag1−/− and TCRβ−/− mice subjected to transient IOP elevation still developed RGC and axon loss, while otherwise immunocompetent GF mice did not, indicating that classical αβ T-cell responses are not responsible for the bulk of neurodegeneration. Second, the timing and nature of immune priming by gut microbiota, or how they may differ between individuals with glaucoma, remains unknown. More work remains to be done to elucidate other roles for microbiota in glaucoma, and to characterize differences in microbiota among patients with glaucomatous disease.
In summary, a real association exists between POAG and active H. pylori infection, but it remains unclear if eradication provides any ocular benefit. Evidence in mice indicates that intestinal dysbiosis may contribute to glaucoma progression by inducing immune intolerance to cross-reactive retinal antigens, but these findings will need to be corroborated in humans. Small observational studies have found inconsistent associations between POAG and periodontal disease.
Concluding remarks
Many of these studies have significant limitations. Because gut and other microbiota are sensitive to diet, environment, and other aspects of the life history, observational studies cannot control for all possible confounders. Studies may be inadvertently using gut microbiota as a proxy for socioeconomic status, diet quality, or environmental exposures. A standardized approach has been proposed, using parallel clinical and translational research in both patients and germ-free mice, to better establish causality in microbiota research.[45] Successful trials are also a nice way to establish causation, but interventions to modify gut flora lag behind the techniques used to characterize them in sophistication and subtlety (see Table 1). Probiotics and prebiotics have mixed evidence of efficacy[88,89] and are difficult to standardize. Heterologous fecal microbiota transplantation has strong evidence of efficacy in certain conditions (e.g. recurrent Clostridoides difficile infection),[90] but is even harder to standardize, with high variance in outcomes based on donor stool quality.[91] Administration of antibiotics in the absence of specific pathogens can only reduce diversity and is not safe. On the horizon are several emerging methods that may provide new ways to leverage discoveries about microbial symbionts in disease. Administering postbiotics, i.e. microbe-derived products rather than the live organism, may avoid bioavailability issues and be easier to standardize; this approach was discussed above in the context of diabetic retinopathy. Microbial strains have been engineered both to efficiently colonize the gut and to express disease-modifying pathways.[92] Finally, bacteriophage therapy[93] or CRISPR-Cas9-based methods[94] may allow the microbiome to be edited in situ without needing to introduce new species.
Table 1.
Interventions proposed to alter gut microbes for disease modification.
| Intervention | Strengths | Limitations |
|---|---|---|
| Antibiotics45 | Effective in suppressing susceptible organisms | Unlikely to promote the growth of desirable organisms, reduces diversity, contributes to antimicrobial resistance |
| Probiotics/prebiotics88,89 | Widely used, safe | Most preparations fail to colonize the intestines, hard to standardize |
| Fecal microbiota transplantation90,91 | Proven effective in some conditions | Donor-dependent, resource intensive |
| “Postbiotics” (bacterial products)34,35,45 | Easier to standardize | Unclear efficacy |
| Engineered bacterial strains92 | Possibly more reliable colonization, controllable | Complex, untested |
| Phage/CRISPR-Cas9 modification93,94 | Targeted introduction or ablation of specific genes or pathways in situ | Complex, untested |
Now research on gut microbiota and ocular health faces an exciting prospect. Many connections have been uncovered between gut microbiota and several chronic eye diseases, but much work remains to be done to elucidate these connections with increasing specificity and certainty. Most importantly, prospective studies need to determine if intervening on gut dysbiosis has a meaningful effect on the prognosis of ocular diseases. Some of these mechanisms may prove to be the foundation of new treatments, and basic and clinical research will need to evolve together to realize the potential of these discoveries in this rapidly changing field.
Funding Sources
Funding was provided in part by NIH grants EY021752, EY024564, and American Diabetes Association grants to QL, and NIH P30 EY02172 and Research to Prevent Blindness grants to the University of Florida. Funders had no role in study design or manuscript preparation.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
References
- 1.Sender R, Fuchs S, Milo R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell. 2016. Jan;164(3):337–40. [DOI] [PubMed] [Google Scholar]
- 2.Matijašić M, Meštrović T, Čipčić Paljetak H, Perić M, Barešić A, Verbanac D. Gut Microbiota beyond Bacteria—Mycobiome, Virome, Archaeome, and Eukaryotic Parasites in IBD. Int J Mol Sci. 2020. Apr;21(8):2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cani PD, Van Hul M, Lefort C, Depommier C, Rastelli M, Everard A. Microbial regulation of organismal energy homeostasis. Nat Metab. 2019. Jan;1(1):34–46. [DOI] [PubMed] [Google Scholar]
- 4.Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020. Jun;30(6):492–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science (80-). 2016. Apr;352(6285):539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hacquard S, Garrido-Oter R, González A, Spaepen S, Ackermann G, Lebeis S, et al. Microbiota and Host Nutrition across Plant and Animal Kingdoms. Cell Host Microbe. 2015. May;17(5):603–16. [DOI] [PubMed] [Google Scholar]
- 7.Dave M, Higgins PD, Middha S, Rioux KP. The human gut microbiome: current knowledge, challenges, and future directions. Transl Res. 2012. Oct;160(4):246–57. [DOI] [PubMed] [Google Scholar]
- 8.Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017. Jun;474(11):1823–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao D, Cho J, Kim MH, Guallar E. The Association of Blood Pressure and Primary Open-Angle Glaucoma: A Meta-analysis. Am J Ophthalmol. 2014. Sep;158(3):615–627.e9. [DOI] [PubMed] [Google Scholar]
- 10.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, et al. Gut Dysbiosis Is Linked to Hypertension. Hypertension. 2015. Jun;65(6):1331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao D, Cho J, Kim MH, Friedman DS, Guallar E. Diabetes, Fasting Glucose, and the Risk of Glaucoma. Ophthalmology. 2015. Jan;122(1):72–8. [DOI] [PubMed] [Google Scholar]
- 12.Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, et al. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020. Jan;51:102590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dehner C, Fine R, Kriegel MA. The microbiome in systemic autoimmune disease: mechanistic insights from recent studies. Curr Opin Rheumatol. 2019. Mar;31(2):201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavuoto KM, Banerjee S, Galor A. Relationship between the microbiome and ocular health. Ocul Surf. 2019. Jul;17(3):384–92. [DOI] [PubMed] [Google Scholar]
- 15.Floyd JL, Grant MB. The Gut–Eye Axis: Lessons Learned from Murine Models. Ophthalmol Ther. 2020. Sep;9(3):499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowan S, Taylor A. The Role of Microbiota in Retinal Disease. 2018; pp 429–35. [DOI] [PubMed]
- 17.Tsirouki T, Dastiridou A, Symeonidis C, Tounakaki O, Brazitikou I, Kalogeropoulos C, et al. A Focus on the Epidemiology of Uveitis. Ocul Immunol Inflamm. 2018. Jan;26(1):2–16. [DOI] [PubMed] [Google Scholar]
- 18.Amador-Patarroyo M, Cristina Peñaranda A, Teresa Bernal M. Autoimmune uveitis. In: Anaya J, Shoenfeld Y, Rojas-Villarraga A, editors. Autoimmunity: From Bench to Bedside [Internet]. Bogota (Columbia); 2013. [Google Scholar]
- 19.Huang X, Ye Z, Cao Q, Su G, Wang Q, Deng J, et al. Gut Microbiota Composition and Fecal Metabolic Phenotype in Patients With Acute Anterior Uveitis. Investig Opthalmology Vis Sci. 2018. Mar;59(3):1523. [DOI] [PubMed] [Google Scholar]
- 20.Kalyana Chakravarthy S, Jayasudha R, Sai Prashanthi G, Ali MH, Sharma S, Tyagi M, et al. Dysbiosis in the Gut Bacterial Microbiome of Patients with Uveitis, an Inflammatory Disease of the Eye. Indian J Microbiol. 2018. Dec;58(4):457–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu X, Chen Y, Chen D. The Role of Gut Microbiome in Autoimmune Uveitis. Ophthalmic Res. 2020. Jul DOI: 10.1159/000510212 [DOI] [PubMed] [Google Scholar]
- 22.Rosenbaum JT, Asquith M. The microbiome and HLA-B27-associated acute anterior uveitis. Nat Rev Rheumatol. 2018. Dec;14(12):704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura YK, Metea C, Karstens L, Asquith M, Gruner H, Moscibrocki C, et al. Gut Microbial Alterations Associated With Protection From Autoimmune Uveitis. Investig Opthalmology Vis Sci. 2016. Jul;57(8):3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhuang Z, Wang Y, Zhu G, Gu Y, Mao L, Hong M, et al. Imbalance of Th17/Treg cells in pathogenesis of patients with human leukocyte antigen B27 associated acute anterior uveitis. Sci Rep. 2017. Mar;7(1):40414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horai R, Zárate-Bladés CR, Dillenburg-Pilla P, Chen J, Kielczewski JL, Silver PB, et al. Microbiota-Dependent Activation of an Autoreactive T Cell Receptor Provokes Autoimmunity in an Immunologically Privileged Site. Immunity. 2015. Aug;43(2):343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leasher JL, Bourne RRA, Flaxman SR, Jonas JB, Keeffe J, Naidoo K, et al. Global Estimates on the Number of People Blind or Visually Impaired by Diabetic Retinopathy: A Meta-analysis From 1990 to 2010. Diabetes Care. 2016. Sep;39(9):1643–9. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Saaddine JB, Chou C-F, Cotch MF, Cheng YJ, Geiss LS, et al. Prevalence of Diabetic Retinopathy in the United States, 2005–2008. JAMA. 2010. Aug;304(6):649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rein DB. The Economic Burden of Major Adult Visual Disorders in the United States. Arch Ophthalmol. 2006. Dec;124(12):1754. [DOI] [PubMed] [Google Scholar]
- 29.Moubayed NM, Bhat RS, Al Farraj D, Dihani N Al, El Ansary A, Fahmy RM. Screening and identification of gut anaerobes (Bacteroidetes) from human diabetic stool samples with and without retinopathy in comparison to control subjects. Microb Pathog. 2019. Apr;129:88–92. [DOI] [PubMed] [Google Scholar]
- 30.Das T, Jayasudha R, Chakravarthy S, Prashanthi GS, Bhargava A, Tyagi M, et al. Alterations in the gut bacterial microbiome in people with type 2 diabetes mellitus and diabetic retinopathy. Sci Rep. 2021. Dec;11(1):2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015. Dec;528(7581):262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang Y, Wang Z, Ma H, Ji S, Chen Z, Cui Z, et al. Dysbiosis and Implication of the Gut Microbiota in Diabetic Retinopathy. Front Cell Infect Microbiol. 2021. Mar;11. DOI: 10.3389/fcimb.2021.646348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beli E, Yan Y, Moldovan L, Vieira CP, Gao R, Duan Y, et al. Restructuring of the Gut Microbiome by Intermittent Fasting Prevents Retinopathy and Prolongs Survival in db/db Mice. Diabetes. 2018. Sep;67(9):1867–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C, Yuan J, Qin D, Gu J, Zhao B, Zhang L, et al. Protection of tauroursodeoxycholic acid on high glucose-induced human retinal microvascular endothelial cells dysfunction and streptozotocin-induced diabetic retinopathy rats. J Ethnopharmacol. 2016. Jun;185:162–70. [DOI] [PubMed] [Google Scholar]
- 35.Gaspar JM, Martins A, Cruz R, Rodrigues CMP, Ambrósio AF, Santiago AR. Tauroursodeoxycholic acid protects retinal neural cells from cell death induced by prolonged exposure to elevated glucose. Neuroscience. 2013. Dec;253:380–8. [DOI] [PubMed] [Google Scholar]
- 36.Oshitari T, Bikbova G, Yamamoto S. Increased expression of phosphorylated c-Jun and phosphorylated c-Jun N-terminal kinase associated with neuronal cell death in diabetic and high glucose exposed rat retinas. Brain Res Bull. 2014. Feb;101:18–25. [DOI] [PubMed] [Google Scholar]
- 37.Zhu L, Wang W, Xie T, Zou J, Nie X, Wang X, et al. TGR5 receptor activation attenuates diabetic retinopathy through suppression of RhoA/ROCK signaling. FASEB J. 2020. Mar;34(3):4189–203. [DOI] [PubMed] [Google Scholar]
- 38.Fernández-Sánchez L, Bravo-Osuna I, Lax P, Arranz-Romera A, Maneu V, Esteban-Pérez S, et al. Controlled delivery of tauroursodeoxycholic acid from biodegradable microspheres slows retinal degeneration and vision loss in P23H rats. PLoS One. 2017. May;12(5):e0177998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein RJ. Complement Factor H Polymorphism in Age-Related Macular Degeneration. Science (80-). 2005. Apr;308(5720):385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee AY, Kulkarni M, Fang AM, Edelstein S, Osborn MP, Brantley MA. The effect of genetic variants in SERPING1 on the risk of neovascular age-related macular degeneration. Br J Ophthalmol. 2010. Jul;94(7):915–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boekhoorn SS. C-reactive Protein Level and Risk of Aging Macula Disorder. Arch Ophthalmol. 2007. Oct;125(10):1396. [DOI] [PubMed] [Google Scholar]
- 42.Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA. 1994. Nov;272(18):1413–20. [PubMed] [Google Scholar]
- 43.SanGiovanni JP, Chew EY, Clemons TE, Davis MD, Ferris FL, Gensler GR, et al. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS Report No. 20. Arch Ophthalmol (Chicago, Ill 1960). 2007. May;125(5):671–9. [DOI] [PubMed] [Google Scholar]
- 44.Watstein DM, McNerney MP, Styczynski MP. Precise metabolic engineering of carotenoid biosynthesis in Escherichia coli towards a low-cost biosensor. Metab Eng. 2015;31:171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2020. Sep DOI: 10.1038/s41579-020-0433-9 [DOI] [PubMed] [Google Scholar]
- 46.Lynch SV, Pedersen O The Human Intestinal Microbiome in Health and Disease. N Engl J Med. 2016. Dec;375(24):2369–79. [DOI] [PubMed] [Google Scholar]
- 47.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014. Jan;505(7484):559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zinkernagel MS, Zysset-Burri DC, Keller I, Berger LE, Leichtle AB, Largiadèr CR, et al. Association of the Intestinal Microbiome with the Development of Neovascular Age-Related Macular Degeneration. Sci Rep. 2017;7(1):40826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zysset-Burri DC, Keller I, Berger LE, Largiadèr CR, Wittwer M, Wolf S, et al. Associations of the intestinal microbiome with the complement system in neovascular age-related macular degeneration. npj Genomic Med. 2020. Dec;5(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu A, Chang J, Lin Y, Shen Z, Bernstein PS. Long-chain and very long-chain polyunsaturated fatty acids in ocular aging and age-related macular degeneration. J Lipid Res. 2010. Nov;51(11):3217–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bui BV, Hu RG, Acosta ML, Donaldson P, Vingrys AJ, Kalloniatis M. Glutamate metabolic pathways and retinal function. J Neurochem. 2009. Oct;111(2):589–99. [DOI] [PubMed] [Google Scholar]
- 52.Andriessen EM, Wilson AM, Mawambo G, Dejda A, Miloudi K, Sennlaub F, et al. Gut microbiota influences pathological angiogenesis in obesity-driven choroidal neovascularization. EMBO Mol Med. 2016. Dec;8(12):1366–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rowan S, Jiang S, Korem T, Szymanski J, Chang M-L, Szelog J, et al. Involvement of a gut–retina axis in protection against dietary glycemia-induced age-related macular degeneration. Proc Natl Acad Sci. 2017. May;114(22):E4472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pennesi ME, Neuringer M, Courtney RJ. Animal models of age related macular degeneration. Mol Aspects Med. 2012. Aug;33(4):487–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ho EXP, Cheung CMG, Sim S, Chu CW, Wilm A, Lin CB, et al. Human pharyngeal microbiota in age-related macular degeneration. PLoS One. 2018. Aug;13(8):e0201768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Potempa M, Potempa J, Kantyka T, Nguyen K-A, Wawrzonek K, Manandhar SP, et al. Interpain A, a Cysteine Proteinase from Prevotella intermedia, Inhibits Complement by Degrading Complement Factor C3. PLoS Pathog. 2009. Feb;5(2):e1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013. Nov;2. DOI: 10.7554/eLife.01202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rullo J, Far PM, Quinn M, Sharma N, Bae S, Irrcher I, et al. Local oral and nasal microbiome diversity in age-related macular degeneration. Sci Rep. 2020. Dec;10(1):3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romano C, Barrett DA, Li Z, Pestronk A, Wax MB. Anti-rhodopsin antibodies in sera from patients with normal-pressure glaucoma. Invest Ophthalmol Vis Sci. 1995. Sep;36(10):1968–75. [PubMed] [Google Scholar]
- 60.Tezel G, Edward DP, Wax MB. Serum Autoantibodies to Optic Nerve Head Glycosaminoglycans in Patients With Glaucoma. Arch Ophthalmol. 1999;117(7):917–24. [DOI] [PubMed] [Google Scholar]
- 61.Yang J, Patil RV, Yu H, Gordon M, Wax MB. T cell subsets and sIL-2R/IL-2 levels in patients with glaucoma. Am J Ophthalmol. 2001. Apr;131(4):421–6. [DOI] [PubMed] [Google Scholar]
- 62.Nayyar A, Gindina S, Barron A, Hu Y, Danias J. Do epigenetic changes caused by commensal microbiota contribute to development of ocular disease? A review of evidence. Hum Genomics. 2020. Dec;14(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baim AD, Movahedan A, Farooq AV, Skondra D. The microbiome and ophthalmic disease. Exp Biol Med. 2019. Apr;244(6):419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Henein C, Khaw PT. The interplay between inflammation, immunity and commensal microflora in glaucomatous neurodegeneration. Ann Eye Sci. 2019. Mar;4:10–10. [Google Scholar]
- 65.Gong H, Zhang S, Li Q, Zuo C, Gao X, Zheng B, et al. Gut microbiota compositional profile and serum metabolic phenotype in patients with primary open-angle glaucoma. Exp Eye Res. 2020. Feb;191:107921. [DOI] [PubMed] [Google Scholar]
- 66.Cicciù M Neurodegenerative Disorders and Periodontal Disease: Is There a Logical Connection? Neuroepidemiology. 2016;47(2):94–5. [DOI] [PubMed] [Google Scholar]
- 67.Hubens WHG, Mohren RJC, Liesenborghs I, Eijssen LMT, Ramdas WD, Webers CAB, et al. The aqueous humor proteome of primary open angle glaucoma: An extensive review. Exp Eye Res. 2020. Aug;197:108077. [DOI] [PubMed] [Google Scholar]
- 68.Shibuya E, Meguro A, Ota M, Kashiwagi K, Mabuchi F, Iijima H, et al. Association of Toll-like Receptor 4 Gene Polymorphisms with Normal Tension Glaucoma. Investig Opthalmology Vis Sci. 2008. Oct;49(10):4453. [DOI] [PubMed] [Google Scholar]
- 69.Astafurov K, Elhawy E, Ren L, Dong CQ, Igboin C, Hyman L, et al. Oral Microbiome Link to Neurodegeneration in Glaucoma. PLoS One. 2014. Sep;9(9):e104416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Polla D, Astafurov K, Hawy E, Hyman L, Hou W, Danias J. A Pilot Study to Evaluate the Oral Microbiome and Dental Health in Primary Open-Angle Glaucoma. J Glaucoma. 2017. Apr;26(4):320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pasquale LR, Hyman L, Wiggs JL, Rosner BA, Joshipura K, McEvoy M, et al. Prospective Study of Oral Health and Risk of Primary Open-Angle Glaucoma in Men. Ophthalmology. 2016. Nov;123(11):2318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun K-T, Shen T-C, Chen S-C, Chang C-L, Li C, Li X, et al. Periodontitis and the subsequent risk of glaucoma: results from the real-world practice. Sci Rep. 2020. Dec;10(1):17568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kountouras J, Mylopoulos N, Boura P, Bessas C, Chatzopoulos D, Venizelos J, et al. Relationship between Helicobacter pylori infection and glaucoma. Ophthalmology. 2001. Mar;108(3):599–604. [DOI] [PubMed] [Google Scholar]
- 74.Zullo A, Ridola L, Hassan C, Bruzzese V, Papini F, Vaira D. Glaucoma and Helicobacter pylori: Eyes wide shut? Dig Liver Dis. 2012. Aug;44(8):627–8. [DOI] [PubMed] [Google Scholar]
- 75.Zeng J, Liu H, Liu X, Ding C. The Relationship Between Helicobacter pylori Infection and Open-Angle Glaucoma: A Meta-Analysis. Investig Opthalmology Vis Sci. 2015. Aug;56(9):5238. [DOI] [PubMed] [Google Scholar]
- 76.Doulberis M, Papaefthymiou A, Polyzos SA, Bargiotas P, Liatsos C, Srivastava DS, et al. Association between Active Helicobacter pylori Infection and Glaucoma: A Systematic Review and Meta-Analysis. Microorganisms. 2020. Jun;8(6):894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kountouras J, Mylopoulos N, Konstas AGP, Zavos C, Chatzopoulos D, Boukla A. Increased levels of Helicobacter pylori IgG antibodies in aqueous humor of patients with primary open-angle and exfoliation glaucoma. Graefe’s Arch Clin Exp Ophthalmol. 2003. Nov;241(11):884–90. [DOI] [PubMed] [Google Scholar]
- 78.Zavos C, Kountouras J, Sakkias G, Venizelos I, Deretzi G, Arapoglou S. Histological Presence of Helicobacter pylori Bacteria in the Trabeculum and Iris of Patients with Primary Open-Angle Glaucoma. Ophthalmic Res. 2012;47(3):150–6. [DOI] [PubMed] [Google Scholar]
- 79.Logan R Adherence of Helicobacter pylori. Aliment Pharmacol Ther. 1996. Apr;10(Sup1):3–15. [DOI] [PubMed] [Google Scholar]
- 80.Ala S, Maleki I, Sanjari Araghi A, Sahebnasagh A, Shahraki A. Helicobacter pylori eradication in the management of glaucoma. Casp J Intern Med. 2020;11(2):143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kountouras J, Mylopoulos N, Chatzopoulos D, Zavos C, Boura P, Konstas AGP, et al. Eradication of Helicobacter pylori May Be Beneficial in the Management of Chronic Open-Angle Glaucoma. Arch Intern Med. 2002. Jun;162(11):1237. [DOI] [PubMed] [Google Scholar]
- 82.Chen H-Y, Lin C-L, Chen W-C, Kao C-H. Does Helicobacter pylori Eradication Reduce the Risk of Open Angle Glaucoma in Patients With Peptic Ulcer Disease? Medicine (Baltimore). 2015. Sep;94(39):e1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tezel G, Seigel GM, Wax MB. Autoantibodies to small heat shock proteins in glaucoma. Invest Ophthalmol Vis Sci. 1998. Nov;39(12):2277–87. [PubMed] [Google Scholar]
- 84.Tezel G, Wax MB. The Mechanisms of hsp27 Antibody-Mediated Apoptosis in Retinal Neuronal Cells. J Neurosci. 2000. May;20(10):3552–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tezel G Immunostaining of Heat Shock Proteins in the Retina and Optic Nerve Head of Normal and Glaucomatous Eyes. Arch Ophthalmol. 2000. Apr;118(4):511. [DOI] [PubMed] [Google Scholar]
- 86.van Eden W, Jansen MAA, Ludwig I, van Kooten P, van der Zee R, Broere F. The Enigma of Heat Shock Proteins in Immune Tolerance. Front Immunol. 2017. Nov;8. DOI: 10.3389/fimmu.2017.01599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen H, Cho K-S, Vu THK, Shen C-H, Kaur M, Chen G, et al. Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma. Nat Commun. 2018;9(1):3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Su GL, Ko CW, Bercik P, Falck-Ytter Y, Sultan S, Weizman AV., et al. AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology. 2020. Aug;159(2):697–705. [DOI] [PubMed] [Google Scholar]
- 89.Kristensen NB, Bryrup T, Allin KH, Nielsen T, Hansen TH, Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med. 2016. Dec;8(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, et al. Duodenal Infusion of Donor Feces for Recurrent Clostridium difficile. N Engl J Med. 2013. Jan;368(5):407–15. [DOI] [PubMed] [Google Scholar]
- 91.Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra AV., Udayappan SD, et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017. Oct;26(4):611–619.e6. [DOI] [PubMed] [Google Scholar]
- 92.Duan FF, Liu JH, March JC. Engineered Commensal Bacteria Reprogram Intestinal Cells Into Glucose-Responsive Insulin-Secreting Cells for the Treatment of Diabetes. Diabetes. 2015. May;64(5):1794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Górski A, Bollyky PL, Przybylski M, Borysowski J, Międzybrodzki R, Jończyk-Matysiak E, et al. Perspectives of Phage Therapy in Non-bacterial Infections. Front Microbiol. 2019. Jan;9. DOI: 10.3389/fmicb.2018.03306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ramachandran G, Bikard D. Editing the microbiome the CRISPR way. Philos Trans R Soc B Biol Sci. 2019. May;374(1772):20180103. [DOI] [PMC free article] [PubMed] [Google Scholar]


