Abstract
Introduction:
Clinical trials typically involve random assignment to treatment conditions. However, random assignment does not guarantee a lack of systematic variation in the outcomes, and application of covariation methods for multiple dependent measures requires complicated assumptions that are often not met.
Methods:
This study employed matched correspondence analysis (CA) for controlling systematic variation and handling multiple outcomes. One hundred nine children with autism spectrum disorder (ASD) were assessed for anxiety symptom severity across four studies, where participants were randomly assigned to either cognitive behavioral therapy (CBT) or treatment as usual or waitlist (TAU/WT). Matched CA is designed to optimally scale only the differences between baseline and posttreatment, rendering the systematic baseline carryover effects irrelevant.
Results:
Differences in treatment efficacy were observed. CBT showed treatment efficacy on anxiety severity and anxiety-related impairment relative to TAU/WT, after the control of baseline carryover effects.
Conclusion:
This study provides a way to control systematic variation between groups at the outset of treatment trials and is expected to provide a novel pathway to more proper assessment of treatment efficacy for children with ASD and anxiety.
Keywords: autism spectrum disorder (ASD), cognitive behavioral therapy (CBT), treatment as usual (TAU), matched correspondence analysis
Introduction
Autism spectrum disorder (ASD) is a childhood onset neurodevelopmental disorder characterized by deficits in social communication, repetitive behaviors, and focused and restricted interests and activities (APA, 2013). High levels of externalizing problems such as hyperactivity (Attwood, 1998) and behavior problems (Green et al., 2000) have also been observed. Children with ASD are often diagnosed with comorbid psychiatric conditions (e.g., anxiety or depressive disorders; Pizzagalli, 2014) or subdiagnostic threshold psychiatric symptoms (e.g., anxious or dysphoric symptoms; de Bruin et al., 2007; Gadow et al., 2009; Kuusikko et al., 2008). The presence of comorbid anxiety disorders in ASD is associated with additional disability across various domains of functioning (e.g., Kaat et al., 2013; van Steensel et al., 2011; Ung et al., 2013; van Steensel & Heeman, 2017). Anxiety is specifically related with higher functional impairment in children, even after accounting for core ASD symptoms, including greater impairment in social responsiveness and social skills (Chang et al., 2012; Green et al., 2000). Peer victimization is also likely to manifest during adolescence for those with ASD (Shtayermman, 2007), which could be a factor associated with higher levels of anxiety. Hence, the treatment of anxiety in children with ASD could produce meaningful benefits, alleviating functional impairment and potentially modestly improving ASD-related impairment (Drahota et al., 2011; Storch et al., 2013).
Regarding anxiety, recent studies have employed randomized controlled trial (RCT) designs to examine treatment efficacy of cognitive-behavioral therapy (CBT) for anxiety in children with ASD (e.g., Kester & Lucyshyn, 2018; Storch et al., 2013; Storch et al., 2015; Storch et al., 2019; Wood et al., 2015; Wood et al., 2020). CBT is considered the first-line treatment for individuals with anxiety disorders with or without ASD. Several trials have used a personalized CBT manual entitled Behavioral Interventions for Anxiety in Children with Autism (Wood et al., 2009), which employs a modular treatment algorithm and includes significant caregiver involvement and training (Ehrenreich-May et al., 2014; Fujii et al., 2013; Storch et al., 2013; Storch et al., 2015; Wood et al., 2009; Wood et al., 2015; Wood et al., 2020). These trials demonstrated significant reductions in anxiety symptoms at post-treatment in comparison with the treatment-as-usual (TAU) and waitlist (WL) conditions, accompanied by improved ratings of adaptive skills and reduced ASD symptom severity (Drahota et al., 2011; Storch et al., 2013; Wood et al., 2009). For example, Wood et al. (2020) found that CBT designed for children with ASD was more effective in reducing anxiety scores on the primary outcome measure than non-ASD adapted CBT, but both types of CBT were better than TAU in lowering the anxiety scores on the outcome measure.
Present Study Aims
Although recent trials have shown efficacy of CBT for anxiety in children with ASD, it remains to be examined whether or not an individual child’s baseline anxiety severity levels (e.g., mild, moderate, severe) influences the posttreatment improvement levels when efficacy of CBT and TAU/WL is evaluated. In an ideal RCT, there would be no difference in anxiety severity levels at baseline, which is a satisfactory condition when the symptom improvement levels (improved, unchanged, deteriorated) are evaluated at posttreatment, assuming no baseline carryover effect on the posttreatment results. Therefore, in the present study, we employ an innovative analytic method, matched correspondence analysis (CA), to control carryover effect at the posttreatment condition. Matched CA is designed to analyze only the differences between baseline and posttreatment, and even if any inequity at baseline is carried over to the posttreatment measures, the differences (between baseline and posttreatment) would signify the pure improvement or deterioration at posttreatment.
No comparison of symptom severity levels at baseline.
When multiple outcome measures have symptom severity levels (e.g., mild, moderate, and severe), equity in severity levels should be compared at baseline; however, such comparisons for their equity at baseline have never been made in any previous studies because the previous studies were based on the mean comparison (between baseline and posttreatment). If there are symptom (e.g., anxiety) severity level differences found at baseline, there would likely be serial dependency to the posttreatment improvement levels when efficacy of CBT and TAU/WT is evaluated, and such dependency should be controlled (Alosh et al., 2014). Most mean-comparison analyses control for serial dependency through analysis of covariance methods (e.g., Storch et al., 2019; Wood et al., 2020). However, when symptom severity levels exist in the symptom measures, with their numeric mean comparisons (between baseline and posttreatment), one cannot detect the severity level difference at baseline because the mean is estimated by aggregating degrees of symptom severity levels.
Symptom severity levels diffused in the aggregated mean values.
Although random assignment is properly conducted at baseline in terms of equity in outcome mean values across different treatment conditions, such equity in the mean does not guarantee equity in symptom severity levels at baseline. Logically, if there is equity in discretized measures, equality is guaranteed in the mean values because they are estimated with aggregation of discretized measures, whereas equity in the mean values does not guarantee equity in the discretized measures. Therefore, examining the discretized measures would be more sensitive to understand the patient’ s anxiety symptom severity (discretization of continuous measures used to create anxiety symptom severity levels: e.g., Kim et al., 2021; Kim et al., 2020a; Kim et al., 2020b and utility of discretized continuous measures: e.g., Kim & Frisby, 2019; Nishisato, 2007). When the severity levels in the discretized measures are compared and baseline differences are found, while there is no difference detected in their aggregated mean values at baseline, the different severity levels (at baseline) can be carried over to the posttreatment condition. This carryover effect occurring in the severity levels could render invalid results if not controlled, due to unexpected inflation or deflation of outcome.
Clinical utility in discretized measures.
Analysis of continuous measures cannot provide how well or poorly a specific improvement level (e.g., “improved” or “deteriorated”) of anxiety measures are related with gender and treatment conditions that are both categorical variables. Examining such categorical associations would be clinically important to assess treatment efficacy for the symptom improvement measures. For a hypothetical example, after treatment, if CBT in female patients is more highly related with the “improved” level of a specific symptom measure than in male patients, then clinicians may conclude that CBT would be more effective in female patients for the specified symptom. However, since statistics based on continuous (mean) measures cannot provide such clinically meaningful information, in the present study we needed to discretize the continuous measures and analyze the discretized data to maximize clinical utility.
Severity levels embedded in continuous measures.
It is reasonable to assume that even if most continuous anxiety measures do not have already defined severity levels, they are almost certainly there and can be defined because low scores in the continuous measures represent mid symptoms and high severe symptoms (e.g., Pediatric Anxiety Rating Scale, Child Behavior Checklist, Multidimensional Anxiety Scale for Children, and Social Responsiveness Scale used in the present study). Therefore, when the continuous measures are properly discretized to generate severity levels, one can obtain clinical important information, otherwise not eligible (e.g., Kim et al., 2021; Kim et al., 2020a; Kim et al., 2020b). Assuming our outcomes are discretized into “improved”, “unchanged”, and “deteriorated” conditions, and then if CBT or TAU/WL is related with the “improved” condition after treatment, one can conclude that the symptoms are improved after treatment. Although additional “unchanged” and “deteriorated” categories are included, to study treatment efficacy, considering only the “improved” condition would be sufficient.
Control the baseline carryover effect in the discretized measures.
To control the baseline carryover effect in the discretized data and study uncontaminated symptom improvement (or deterioration) at the treatment condition, we employ matched CA that is aimed to analyze both discretized data at baseline and at posttreatment simultaneously and to detect the differences between baseline and posttreatment. Therefore, this difference information, even if any measures are unfairly inflated/deflated in the severity levels at baseline, irrespective of random assignment, represents true symptom improvement/deterioration after treatment, making the systematic inflation/deflation irrelevant.
Examining the gender interaction with treatment.
We also aimed to examine the gender interaction effect in both treatment conditions (gender x CBT and gender x TAU/WL). Although there are a few studies regarding gender differences in ASD (e.g., Matheis, et al., 2019; Sipes et al., 2011), no studies investigated the gender interaction with treatment for anxiety. This is a clinically meaningful area considering gender differences in manifestation of ASD and associated emotional responses (Rivet & Matson, 2011). Thus, we conduct matched CA for the discretized anxiety symptom measures, with the gender interaction with CBT and TAU/WL.
Method
Participants
The present study analyzed archival data that had ethics approval at the institution where the RCTs occurred. The IRB granted it exemption at the institution of the first author. One hundred nine (77% male) children between the ages of 7–16 years (M = 11.03, SD = 2.21) diagnosed with ASD participated in the varied studies (Storch et al., 2013; Storch et al., 2015; Storch et al., 2019; Wood et al., 2015). The children were identified as 79.8% White; 0.9% African American; 5.5% Asian; 8.3% Latino and Hispanic; and 5.5% Other/Multiracial. The participants had the following primary anxiety disorders; Separation Anxiety Disorder (n = 16, 14.7%); Social Anxiety Disorder (n = 45, 41.3%); GAD (n = 32, 29.4%); OCD (study inclusion criteria followed DSM-IV; n = 14, 12.8%); and two missing data points (n = 2, 1.8%) had anxiety symptoms assessed but did not reach diagnostic threshold for an anxiety disorder. Individuals randomized to CBT (n = 59) or TAU/WL (n = 50), who were on an established medication regime maintained their medication dosage for the duration of participation. Medications (if applicable) were stable at their present dose for 6 (antipsychotics, ADHD medications) or 8 weeks (e.g., antidepressants) prior to study enrollment for all participants, regardless of condition.
Procedures
Services were provided in a university-based, multidisciplinary behavioral health clinic specializing in the treatment of pediatric anxiety in children with and without ASD. The screening assessment was completed over one to two days depending on the specific study. The post-treatment assessment was completed within a week after the 16th therapy session. Responders to CBT completed a follow-up assessment one month following their post-treatment assessment. Clinician-rated measures were administered by trained graduate-level independent evaluators that were blind to an intervention condition.
Measures
Autism spectrum diagnoses were determined at screening via best estimate procedures (Leckman et al., 1982) and differed somewhat between studies. This procedure involved the administration of the Autism Diagnosis Interview–Revised (ADI-R) (Rutter et al., 2003) in all children and either the Childhood Autism Rating Scale (Schopler et al., 1980) or Autism Diagnosis Observation Schedule (Lord et al., 1999) by a certified doctoral-level evaluator. All parent-, child-, and clinician-rated measures were completed at baseline and posttreatment, and at follow-up assessments, unless otherwise noted.
The following ASD and anxiety related measures were used: Pediatric Anxiety Rating Scale (PARS; RUPP, 2002) is a clinician-administered checklist of anxiety symptoms in children and adolescents and PARS total score inter-rater reliability (= 0.86) and test-retest reliability (= 0.83) were good; (2) Clinical Global Impression-Severity (CGI-S) (Guy, 1976) contains a single-item scale of severity in anxiety symptoms that reflects the overall severity of anxiety symptoms and associated interference; since this is a single item-measurement, reliability estimate was not available; Multidimensional Anxiety Scale for Children (MASC; March et al., 1997) is administered as a part of anxiety symptom relevant measures, covering physical symptoms, social anxiety, separation anxiety and panic, and harm avoidance, and their internal consistency as a measure of reliability were sound (e.g., α = 0.90; see March et al., 1997; Wei et al., 2014); Social Responsiveness Scale (SRS; Constantino, 2002) is designed to assess children’ s autism–specific characteristics such as social awareness, social information processing, capacity for reciprocal social communication, social anxiety/avoidance, and autistic preoccupations and traits, demonstrating strong internal consistency as a reliability measure (e.g., α = 0.91 – 0.97; see Bölte et al., 2008 for details); and Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2001) is designed to assess behavioral and emotional problems in school-age children (ages 6-18) and its internal consistency was α = 0.80 and test-retest reliability was 0.88 (Achenbach & Rescorla, 2001). Pandolfi et al. (2012) showed that the fit measures for a two-factor model defined with Internalizing and Externalizing factors was supported, and the present study used externalizing and internalizing domain scores.
Discretization of continuous measures.
To conduct matched CA, we discretize total scores for PARS, MASC, CBCL–Internalizing, CBCL–Externalizing, and SRS. With Z-scores of total scores for these measures, one standard deviation (of Z-scores) equal to or below the mean of zero (Z ≤ −1) are assigned as “1 = mild (at baseline) or improved (at posttreatment)”. Within one standard deviation (−1 < Z < −1) are assigned as “2 = moderate (at baseline) or unchanged (at posttreatment)”, and one standard deviation above the mean (Z ≥ +1) were assigned as “3 = severe (at baseline) or deteriorated (at posttreatment).” This discretization has been used in the previous studies (e.g., Kim et al., 2020a; Kim et al., 2020b; Kim & Frisby, 2019). We re-categorized the CGI-S that was initially categorically coded (e.g., mild, moderate, moderate-severe, and severe) into the same severity (at baseline) and improvement (at posttreatment) levels as well.
Gender.
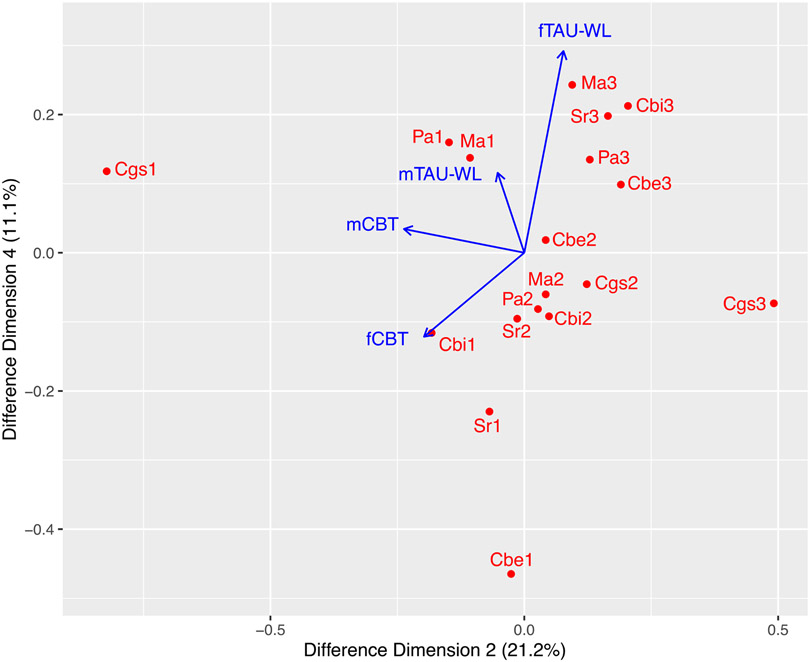
We examined the gender effect on the primary outcome measures. However, different from the previous study (e.g., Wood et al., 2020), we examined the effect of gender interacted with CBT and TAU/WL and thus, gender was interactively coded with CBT and TAU/WL such as female/male CBT and female/male TAU/WL. This gender-interactive coding for CBT and TAU/WL is included in the results (e.g., see Figure 1).
Figure 1. A Difference 2-D Map: Baseline Controlled.
Note. m = male and f = female. 1 = improved, 2 = moderate, and 3 = severe. Cbi = CBCL-Internalizing, Cbe = CBCL-Externalizing, Ma = MASC, Sr = SRS, Pa = PARS, Cgs = CGI-Severity.
Treatment
Cognitive-behavioral therapy (CBT).
Fifty-nine participants (n = 59) were randomly assigned and received 16 weekly, individual CBT sessions (60 – 90 minutes) according to a developmentally modified version of the Behavioral Interventions for Anxiety in Children with Autism treatment manual (Wood & Drahota, 2005; Wood et al., 2009). For each child, a minimum of three sessions were spent developing coping skills (e.g., behavioral activation, cognitive restructuring) with at least eight sessions of exposure therapy. Beyond these core components, additional modules were implemented as needed to address social and adaptive skill deficits/problems, poor motivation, social/school issues, and comorbid conditions. For all children, treatment was provided by Ph.D. students and postdoctoral fellows in clinical psychology with a termination module that addressed relapse prevention and continuing treatment progress. Adherence was checked through review of randomly selected CBT sessions in terms of correspondence with planned session content as well as session checklists. Clinical supervision with a clinical psychologist was held weekly.
Treatment-as-usual (TAU)/Waitlist (WL).
Fifty participants (n = 50) were randomly assigned as part of their respective study to TAU (n = 36) or WL (n = 14). In TAU, participants continued with preexisting medications or therapy and were able to initiate dosage changes, new medications with the prescribing provider or initiate therapy. In the WL arm, subjects did not initiate new treatment but remained on medication at the dose at baseline. The Service Assessment for Children and Adolescents—Service Use Scale was completed at the post- assessment to assess the types of services utilized by participants in the TAU or WL condition. We combined these two conditions, labeled as TAU/WL.
Analysis Plan
The difference 2-dimenional map.
We first construct a 2-dimenaional (or hereafter 2-D) map with the first two dimensions (that accounted for the largest amount of total variance) estimated from the differences in ASD related-anxiety symptom measures between baseline and posttreatment (where baseline was controlled). This difference 2-D map is intended to visually inspect category associations without any numerical statistics. The visual inspection is easy to comprehend the category associations, but to scrutinize the visual inspection results, we estimated the visually inspected configurations with correlations, using the estimation method introduced by Kim and Grochowalski (2019).
Biased results from related measures.
Previous studies have utilized analysis of covariance (ANCOVA) for continuous outcome measures such as PARS, CBCL, MASC, and SRS (e.g., Storch et al., 2010, 2013, 2015 and Wood et al., 2015). However, if the measuring variables were correlated, it is possible that separate analysis may cause biased results. For the present study, we examined the correlations between the same symptom measures, such as PARS, MASC, CBCL, SRS, CGI-Severity, used in the previous studies (by Storch et al., 2015 and Wood et al., 2015) and they were significantly related, and the correlations were between r = 0.41 to r = 0.74 (all p-values < 0.001). Matched CA analyzes all related variables simultaneously and carries no statistical biases (e.g., Kim & Annunziato, 2020; Kim et al, 2020a; Kim et al, 2020b).
Matched Correspondence Analysis (CA)
Applying matched CA to repeated measures.
The original matched CA paradigm was designed to compare two between-group matched matrices (with the same row and column entities), such as female versus male, to see on which aspects females and males tend to agree or differ most (Greenacre, 2003; 2017). There is a fundamental difference between the original one and the current one employed here. We apply the matched CA paradigm to the analysis of a related sample, where the same individuals were measured twice at baseline and posttreatment. Matched CA of the repeated measures allows us to examine to what extent improvement presents after treatment. However, in this study we had to sacrifice the matching between individual cases; rather, we matched the treatment conditions. Unlike other CA variants, matched CA estimates two types of dimensions; sum dimensions and difference dimensions. Although our main interest was to study the differences between repeated measures, we included both the sum and difference dimensional results for comparison because the sum dimensional results actually represented the results in which the baseline systematic variation was not controlled.
Interpreting symptom points in a difference 2-D map.
A 2-D map includes configurations of the post-treatment – baseline differences in both severity levels of anxiety symptom measures and gender x treatment conditions. If the points and lines are away from the origin of (0, 0), this implies variance changes (either in improvement or deterioration) after treatment. To visually comprehend magnitudes of category associations in a 2-D map, it is important to examine angles between categories because angles (actually consign angles) between categories are directly related with correlations: If an angle between categories is close to 0°, a magnitude of correlation would be close to +1.00. Visual inspection is convenient to approximate category associations, but a decision based on solely visual inspection may be arbitrary. Therefore, we will estimate the visually inspected category associations with correlations, utilizing the method introduced by Kim and Grochowalski (2019)
Results
Examine Anxiety Symptom Measures at Baseline and at Posttreatment
No carryover effect in numeric measures.
Satisfied with multivariate normality and homogeneous variance assumptions, we conducted MANOVA to study the group differences between CBT and TAU/WL for total scores of PARS, CBCL–Internalizing, CBCL–Externalizing, MASC, and SRS. At baseline, there was no statistical difference between the treatments for all five symptom measures at α = 0.05. At posttreatment, the mean scores of PARS and SRS for the CBT arm were significantly smaller than the mean scores for the TAU/WL arm at α = 0.01, but not the other three measures. No baseline carryover effect appeared in the continuous posttreatment results.
Carryover effects appearing in discretized anxiety symptom measures.
The discretized version of the same numeric symptom measures was used here. To examine if the baseline severity levels carried over to the posttreatment results, we examined correlations between baseline severity and posttreatment improvement levels. If the baseline severity levels systematically carried over to the posttreatment improvement conditions, the diagonal correlations between baseline severity and posttreatment improvement levels would be strong and positive. In CBT, except PARS, all diagonal correlations were substantial and positive (r ≥ +0.71). In TAU/WT, except two diagonal correlations in PARS and MASC, all other diagonal correlations were larger than or equal to +0.71. In this study we considered only positive correlation equal to or larger than 0.72 because it accounts for at least 50% (0.712 = 0.50) of the shared variance between categories. However, we did not consider any negative correlation irrespective of its magnitude, since the negative correlation measures linear relationships of the opposite (so irrelevant) characteristics of the paired categories. We examined the diagonal correlations of only “mid” at baseline and “improved” at posttreatment. Patients who had “mild” symptoms at baseline would have a high chance to be “improved” in their symptoms at posttreatment: examining the relationships with the “improved” levels is a key to evaluate treatment efficacy (see Table 1).
Table 1.
Baseline-Uncontrolled Correlations between Baseline and Posttreatment Symptom Severity Levels
| CBT | TAU/WT | |||||
|---|---|---|---|---|---|---|
| pPa1 | pPa2 | pPa3 | pPa1 | pPa2 | pPa3 | |
| bPa1 | 0.69 | −0.29 | −0.78 | 0.75 | 0.26 | −0.89 |
| bPa2 | 0.44 | 0.01 | −0.94 | −0.74 | −0.27 | 0.9 |
| bPa3 | −0.56 | 0.12 | 0.88 | −0.21 | 0.97 | −0.84 |
| pCbi1 | pCbi2 | pCbi3 | pCbi1 | pCbi2 | pCbi3 | |
| bCbi1 | 0.89 | −0.25 | −0.64 | 0.99 | −0.48 | −0.03 |
| bCbi2 | −0.1 | 0.81 | −0.95 | −0.69 | 0.93 | −0.6 |
| bCbi3 | −0.36 | −0.45 | 0.99 | −0.31 | −0.77 | 0.99 |
| pCbe1 | pCbe2 | pCbe3 | pCbe1 | pCbe2 | pCbe3 | |
| bCbe1 | 0.98 | −0.53 | −0.32 | 0.71 | 0.43 | −0.83 |
| bCbe2 | −0.69 | 1 | −0.64 | 0.01 | 0.94 | −0.98 |
| bCbe3 | −0.65 | −0.1 | 0.84 | −0.22 | −0.84 | 1 |
| pMa1 | pMa2 | pMa3 | pMa1 | pMa2 | pMa3 | |
| bMa1 | 0.93 | −0.4 | −0.52 | 0.67 | −0.98 | 0.46 |
| bMa2 | −0.27 | 0.87 | −0.9 | −0.66 | 0.98 | −0.47 |
| bMa3 | −0.63 | −0.11 | 0.88 | 0.65 | −0.98 | 0.48 |
| pSr1 | pSr2 | pSr3 | pSr1 | pSr2 | pSr3 | |
| bSr1 | 0.86 | −0.49 | −0.61 | 0.92 | −0.77 | 0.02 |
| bSr2 | −0.59 | 0.92 | −0.73 | −0.68 | 0.96 | −0.43 |
| bSr3 | −0.62 | 0.14 | 0.85 | 0.27 | −0.98 | 0.8 |
Note. The diagonal correlations that were equal to or larger than +0.71 were bolded. b = baseline and p = posttreatment. Pa = PARS; Cbi = CBCL-Internalizing; Cbe = CBCL-Externalizing; Ma = MASC; and Sr = SRS.
Interpreting Category Associations in a 2-D Map
We conducted matched CA to examine both difference dimensions and sum dimensions and depicted a difference 2-D map where the baseline disparity was controlled.
A difference 2-D map.
The difference 2-D map accounted for 32.3% of total variance. As shown in Figure 1, if a hypothetical line is drawn from Cgs1 (improved CGI-Severity) to the origin of (0, 0), this hypothetical line and male CBT (mCBT) was overlapped, indicating their correlation would be +1. On the other hand, mCBT was almost orthogonal to the improved levels of SRS (Sr1) and CBCL–Externalizing (Cbe1), their correlations would be trivial or close to zero. The female CBT (fCBT) was almost overlapped with the improved level of CBCL–Internalizing (Cbi1), implying their correlation would be close to +1 and also adjacent to Sr1 and Cbe1, indicating their correlations would be positive and substantial.
Estimating visually inspected associations with correlations.
To enhance interpretation, we estimated the visually inspected associations with correlations. The correlation estimates are summarized in Table 2 that were consistent with the visual inspection results as shown in Figure 1. There was a perfect correlation between mCBT and Cgs1 (r = +1.00). The correlations of mCBT with Cbi1, Ma1, and Pa1 were: r = +0.81 – r = +0.89. The correlations of mTAU/WL with Ma1 and Pa1 were r = +0.87 and r = +0.83, respectively. The correlations of fCBT with Cbi1, Cbe1, and Sr1 were: r = +0.71 – r = +0.96.
Table 2.
Baseline-Controlled Correlations between Gender x Treatment and Anxiety Symptom Measures in a Difference 2-D Map:
| mCBT | mTAU/WL | fCBT | fTAU/WL | |
|---|---|---|---|---|
| Pa1 | 0.89 | 0.83 | 0.2 | 0.46 |
| Cbi1 | 0.81 | −0.11 | 0.96 | −0.58 |
| Cbe1 | −0.12 | −0.92 | 0.71 | −0.99 |
| Ma1 | 0.85 | 0.87 | 0.12 | 0.53 |
| Sr1 | 0.19 | −0.76 | 0.89 | −0.98 |
| Cgs1 | 1 | 0.41 | 0.69 | −0.09 |
Note. The correlations equal to larger than +0.71 were bolded. We examined only the improved condition (“1”) in the measurement categories. m = male and f = female. 1 = improved, 2 = moderate, and 3 = severe. Cbi = CBCL-Internalizing, Cbe = CBCL-Externalizing, Ma = MASC, Sr = SRS, Pa = PARS, Cgs = CGI-Severity.
Estimating correlations in a sum 2-D map.
We estimated correlations from a sum 2-D map to compare them with the correlations estimated from the difference 2-D map. The correlational results estimated from the sum 2-D map are summarized in Table 3, and these correlations are biased because the baseline disparity was not controlled. These correlational results were quite different from those estimated from the difference 2-D map (see Table 2).
Table 3.
Comparison between Sum and Posttreatment Correlational Patterns in Improved Categories: Baseline Not Controlled
| Sum 2-D Map: Baseline Not Controlled |
Posttreatment 2-D Map: Baseline Not Controlled |
|||||||
|---|---|---|---|---|---|---|---|---|
| mCBT | mTAU/WL | fCBT | fTAU/WL | mCBT | mTAU/WL | fCBT | fTAU/WL | |
| Pa1 | 0.87 | −0.98 | 0.17 | −0.95 | 0.95 | −0.9 | −0.02 | −0.95 |
| Cbi1 | 0.92 | −0.95 | 0.07 | −0.91 | 0.95 | −0.9 | −0.02 | −0.95 |
| Cbe1 | −0.74 | 1.00 | −0.39 | 1.00 | 0.67 | −1 | 0.48 | −0.98 |
| Ma1 | 0.91 | −0.97 | 0.1 | −0.93 | 0.98 | −0.86 | −0.11 | −0.92 |
| Sr1 | 0.86 | −0.99 | 0.2 | −0.96 | 0.71 | −1 | 0.45 | −0.98 |
| Cgs1 | 0.87 | −0.98 | 0.18 | −0.95 | 0.85 | −0.98 | 0.21 | −1 |
Note. We boxed the correlations whose magnitudes and directions were similar between the Sum and Posttreatment conditions among the correlations equal to or larger than +0.71. We examined only the improved condition (“1”) in the measurement categories. m = male and f = female. 1 = improved. Cbi = CBCL-Internalizing, Cbe = CBCL-Externalizing, Ma = MASC, Sr = SRS, Pa = PARS, Cgs = CGI-Severity.
Discussion
Treatment research is often complicated by bias in measures at baseline, even when randomization is conducted given uneven distribution of severity scores (i.e., frequencies in the mild, moderate, and severe categories). The present study is the first one to evaluate treatment efficacy while also controlling for the differences in anxiety symptom severity levels at baseline through the application of matched correspondence analysis.
Correlations estimated from the controlled anxiety severity at baseline.
As shown in the correlational results from the difference 2-D map (see Figure 1 and Table 2), we found that there were highly specific treatment effects for CBT and TAU/WL. We found gender differences in treatment efficacy, with each treatment showing differential efficacy for the symptom measures; for example, male participants in both CBT and TAU/WL showed reduced anxiety symptoms as measured by PARS and MASC, but only female participants in CBT showed reduced anxiety symptoms as measured by SRS and none of the female participants in TAU/WT showed reduced anxiety symptoms. As shown in Table 2, in CBT, more male than female participants were classified into the improved categories of the anxiety symptom measures than in TAU/WL, indicating that CBT outperformed TAU/WL in treatment efficacy. As explained previously, the present study is the first one that examined correlations among severity levels of multiple anxiety measures relevant to children with ASD, in interactions with gender and treatment conditions.
Correlations estimated from uncontrolled anxiety severity at baseline.
The correlations from the sum 2-D map and the posttreatment 2-D map, where the baseline disparity was not controlled in both conditions, were similar and showed that only male participants in CBT were related with improved categories of outcome measures (see Table 3). In these correlation estimates, the baseline severity levels were not controlled and therefore, as hypothesized, such baseline biases were carried over to the posttreatment correlational results, regardless engagement of treatment. We verified these in Table 3. If a researcher relied on the posttreatment results only without controlling the baseline effects in symptom severity, he/she would likely end up with a wrong conclusion; that only males in CBT outperformed other conditions in treatment efficacy. However, this conclusion was based on the biased results in which the baseline effects were not controlled. The matched CA approach proposed in this study handled the baseline biases through optimal scaling of the baseline – post-treatment differences in the symptom measures.
Implications for clinical research and treatment.
Isolating pre-treatment characteristics is a long-standing concern in clinical research. For example, in single-case research, it was long assumed that visual inspection alone would reveal the effects of an intervention. It was only when the serial dependency of each time point on the one that preceded it was isolated was it shown that the pretreatment status significantly biased the conclusions (i.e., Matyas & Greenwood, 1990). The challenge of serial dependency extends to group designs, and as a result, isolating the true treatment effect is a priority in clinical research. As noted earlier, traditional statistical methods are typically underpowered for these analyses, and considering the high costs associated with controlled clinical trials, the application of matched correspondence analysis has the potential to facilitate research on pure treatment effects.
The findings from this study can serve to refine treatment decision trees and other therapy algorithms (i.e., Goodheart et al., 2006; Neufeld, 2007). As clinics move to evidence-based practice, increasingly there is a reliance on treatment decision trees that account for baseline conditions, as well as treatment relevant demographic variables, in crafting interventions. The findings from this research, employing a novel analytic model, has the potential to significantly contribute to the development of empirically-deriven treatment programs.
Limitation.
In the present study we applied the innovative matched CA to control of inequity in anxiety severity at baseline to properly state treatment efficacy at the posttreatment condition. However, matched CA can be utilized with only categorical data and therefore, when data are continuous, a researcher should properly discretize them into a few categories of interest to conduct matched CA, as shown in this study. Also, the matched CA paradigm was built with the R codes, and a researcher should have minimal knowledge of the R language. For replication of our results, we have included data and relevant R codes in Appendix.
Supplementary Material
Highlights.
Differences in symptom severity at baseline carried over to the improvement levels at posttreatment.
After the symptom severity disparity at baseline controlled, the improvement at posttreatment examined between CBT and TAU/WT.
CBT showed treatment efficacy on anxiety severity and anxiety-related impairment.
Acknowledgements
The authors would like to acknowledge the contributions of Adam B. Lewin, Tanya K. Murphy, and Jane P. Mutch.
Funding
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number P50HD103555 for use of the Preclinical and Clinical Outcomes Core facilities. All Children’s Hospital Research Foundation, and University of South Florida Internal Grants Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Drs. Kim and McKay have no conflicts to disclose. Dr. Storch receives royalties from Elsevier, Wiley, Oxford, Lawrence Erlbaum, Springer, and Jessica Kingsley. He receives grant funding from NIH, Texas Higher Education Coordinating Board, the Ream Foundation, and Houston Greater Community Foundation. He has served as a consultant for Levo Therapeutics.
Contributor Information
Se-Kang Kim, Department of Psychology, Fordham University.
Dean McKay, Department of Psychology, Fordham University.
Sandra L. Cepeda, Department of Psychiatry & Behavioral Sciences, Baylor College of Medicine
Sophie C. Schneider, Department of Psychiatry & Behavioral Sciences, Baylor College of Medicine
Jeffrey Wood, Department of Education, University of California, Los Angeles.
Eric A. Storch, Department of Psychiatry & Behavioral Sciences, Baylor College of Medicine
References
- Achenbach TM, & Rescorla LA (2001). Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Alosh M, Bretz F, & Huque M (2014). Advanced multiplicity adjustment methods in clinical trials. Statistics in Medicine, 33, 693–713. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Attwood T (1998). Asperger's Syndrome: A Guide for Parents and Professionals. Philadelphia: Jessica Kingsley. [Google Scholar]
- Bölte S, Poustak F, & Constantino JN (2008). Assessing asutistic tratis: cross-cultural validation of the social response scale (SRS). Autism Research, 1, 354–363. [DOI] [PubMed] [Google Scholar]
- Chang YC, Quan J, & Wood JJ (2012). Effects of anxiety disorder severity on social functioning in children with autism spectrum disorders. Journal of Developmental and Physical Disabilities, 24, 235–245. [Google Scholar]
- Constantino R (2002). The pest termites of South America: taxonomy, distribution and status. Journal of Applied Entomology, 126, 355–365. [Google Scholar]
- de Bruin EI, Ferdinand RF, Meester S, de Nijs PF, & Verheij F (2007). High rates of psychiatric co-morbidity in PDD-NOS. Journal of autism and developmental disorders, 37, 877–886. [DOI] [PubMed] [Google Scholar]
- Drahota A, Wood JJ, Sze KM, & Van Dyke M (2011). Effects of cognitive behavioral therapy on daily living skills in children with high-functioning autism and concurrent anxiety disorders. Journal of autism and developmental disorders, 41, 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich-May J, Storch EA, Queen AH, Rodriguez JH, Ghilain CS, Alessandri M, … Fujii C (2014). An open trial of cognitive-behavioral therapy for anxiety disorders in adolescents with autism spectrum disorders. Focus on Autism and Other Developmental Disabilities, 29, 145–155. 10.1177/1088357614533381 [DOI] [Google Scholar]
- Fujii C, Renno P, McLeod BD, Lin CE, Decker K, Zielinski K, & Wood JJ (2013). Intensive cognitive behavioral therapy for anxiety disorders in school-aged children with autism: A preliminary comparison with treatment-as-usual. School Mental Health, 5, 25–37. [Google Scholar]
- Gadow KD, DeVincent CJ, & Schneider J (2009). Comparative study of children with ADHD only, autism spectrum disorder + ADHD, and chronic multiple tic disorder + ADHD. Journal of Attention Disorders, 12, 474–485. [DOI] [PubMed] [Google Scholar]
- Goodheart CD, Kazdin AE, & Sternberg RJ (2007). Evidence-based psychotherapy: Where practice and research meet. Washington, DC: American Psychological Association. [Google Scholar]
- Green J, Gilchrist A, Burton D, & Cox A (2000). Social and psychiatric functioning in adolescents with Asperger syndrome compared with conduct disorder. Journal of autism and developmental disorders, 30, 279–293. [DOI] [PubMed] [Google Scholar]
- Greenacre MJ (2003). Singular value decomposition of matched matrices. J Appl Stat, 30, pp 1101–1113. 10.1080/026647.6032000107132. [DOI] [Google Scholar]
- Greenacre MJ (2010). Biplots in Practice: Fundación BBVA. [Google Scholar]
- Greenacre MJ (2017). Correspondence Analysis in Practice. 3nd ed. Boca Raton, FL: Chapman & Hall/CRC. [Google Scholar]
- Greenacre M (2016). Data reporting and visualization in ecology. Polar Biology, 39, 2189–2205. [Google Scholar]
- Guy W (1976). Early clinical drug evaluation (ECDEU) assessment manual. Rockville: National Institute of Mental Health. [Google Scholar]
- Horwitz SM, Hoagwood K, Stiffman AR, et al. (2001). Reliability of the services assessment for children and adolescents. Psychiatric Services, 52, 1088–1094. [DOI] [PubMed] [Google Scholar]
- Kaat AJ, Gadow KD, & Lecavalier L (2013). Psychiatric symptom impairment in children with autism spectrum disorders. Journal of Abnormal Child Psychology, 41, 959–969. [DOI] [PubMed] [Google Scholar]
- Kester KR, & Lucyshyn JM (2018). Cognitive behavior therapy to treat anxiety among children with autism spectrum disorders: A systematic review. Research in Autism Spectrum Disorders, 52, 37–50. 10.1016/j.rasd.2018.05.002 [DOI] [Google Scholar]
- Kim S-K (2020). Test treatment effect differences in repeatedly measured symptoms with binary values: The matched correspondence analysis approach. Behavior Research Methods, 52, 148–1490. 10.3758/s13428-019-01328-9 [DOI] [PubMed] [Google Scholar]
- Kim S-K, & Annunziato RA (2020). Can eating disorder treatment also alleviate psychiatric comorbidity: Matched correspondence analysis? Psychotherapy & Psychosomatics. Advance online publication. 10.1159/000505672 [DOI] [PubMed] [Google Scholar]
- Kim S-K, & Annunziato RA (2018). Estimating correlations among cardiovascular patients’ psychiatric and physical symptom indicators: The biplot in correspondence analysis approach. International Journal of Methods in Psychiatric Research. 10.1002/mpr.1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-K, & Frisby CL (2019). Gaining from discretization of continuous data: The correspondence analysis biplot approach. Behavior Research Methods, 51(2), 589–601. 10.3758/s13428-018-1161-1 [DOI] [PubMed] [Google Scholar]
- Kim S-K, & Grochowalski JH (2019). Exploratory visual inspection of category associations and correlation estimation in multidimensional subspaces. Journal of Classification, 36(2), 177–199. 10.1007/s00357-018-9277-7 [DOI] [Google Scholar]
- Kim S-K, McKay D, Goodman WK, Small BJ, McNamara JP, & Murphy TK, & Storch EA (2020a). Anxiety and symptom impact mediate outcome in cognitive-behavior therapy and pharmacotherapy for childhood obsessive-compulsive disorder: The correspondence analysis approach. Journal of Psychopathology and Behavioral Assessment, 42, 739–750. 10.1007/s10862-020-09824-5 [DOI] [Google Scholar]
- Kim S-K, McKay D, Ehrenreich-May J, Wood J, & Storch E (2020b). Assessing treatment efficacy by examining relationships between age groups of children with autism spectrum disorder and clinical anxiety symptoms: Prediction by correspondence analysis. Journal of Affective Disorders, 265(15), 645–650. 10.1016/j.jad.2019.11.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-K, McKay D, Murphy TK, Bussing R, McNamara JP, Goodman WK, & Storch EC (2021). Age moderated–anxiety mediation for multimodal treatment outcome among children with obsessive-compulsive disorder: an evaluation with correspondence analysis. Journal of Affective Disorders, 282, 766–775. 10.1016/j.jad.2020.12.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusikko S, Pollock-Wurman R, Jussila K, Carter AS, Mattila ML, Ebeling H, … & Moilanen I (2008). Social anxiety in high-functioning children and adolescents with autism and Asperger syndrome. Journal of autism and developmental disorders, 38, 1697–1709. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Sholomskas D, Thompson D, Belanger A, & Weissman MM (1982). Best estimate of lifetime psychiatric diagnosis: a methodological study. Archives of general psychiatry, 39, 879–883. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, & Risi S (1999). Autism diagnostic observation schedule-WPS (ADOS-WPS). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, & Conners CK (1997). The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. Journal of the American academy of child & adolescent psychiatry, 36, 554–565. [DOI] [PubMed] [Google Scholar]
- Matheis M, Matson JL, Hong E, & Cervantes PE (2019). Gender differences and similarities: Autism symptomatology and developmental functioning in young children. Journal of Autism and Developmental Disorders, 49(3), 1219–1231. DOI: 10.1007/s10803-018-3819-z [DOI] [PubMed] [Google Scholar]
- Matyas TA, & Greenwood KM (1990). Visual analysis of single-case time series: Effects on variability, serial dependence, and magnitude of intervention effects. Journal of Applied Behavior Analysis, 23, 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld RWJ (2007). Advances in clinical cognitive science: Formal modeling of processes and symptoms. Washington, DC: American Psychological Association. [Google Scholar]
- Nishisato S (2007). Multidimensional Nonlinear Descriptive Analysis. Boca Raton, FL: Chapman & Hall/CRC. [Google Scholar]
- Pandolfi V, Magyar CI, & Dill CA (2012). An initial psychometric evaluation of the CBCL 6-18 in a sample of youth with autism spectrum disorders. Research in Autism Spectrum Disorders, 6, 96–108. doi: 10.1016/j.rasd.2011.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzaballi DA (2014). Depression, Stress, and Anhedonia: Toward a Synthesis and Integrated Model. Annual Review of Clinical Psychology, 10, 393–423. 10.1146/annurev-clinpsy-050212-185606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinck M, Rörtgen T, Lange WG, Dotsch R, Wigboldus DH, & Becker ES (2010). Social anxiety predicts avoidance behaviour in virtual encounters. Cognition and Emotion, 24, 1269–1276. [Google Scholar]
- Rivet TT, & Matson JL (2011). Review of gender differences in core symptomatology in autism spectrum disorders. Research in Autism Spectrum Disorders, 5, 957–976. [Google Scholar]
- RUPP (2002). The pediatric anxiety rating scale (PARS): Development and psychometric properties. Journal of the American Academy of Child & Adolescent Psychiatry, 41, 1061–1069. [DOI] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, & Lord C (2003). Autism diagnostic interview-revised. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Schopler E, Reichler RJ, DeVellis RF, & Daly K (1980). Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS). Journal of autism and developmental disorders, 10(1), 91–103. [DOI] [PubMed] [Google Scholar]
- Shtayermman O (2007). Peer victimization in adolescents and young adults diagnosed with Asperger's syndrome: A link to depressive symptomatology, anxiety symptomatology and suicidal ideation. Issues in comprehensive pediatric nursing, 30, 87–107. [DOI] [PubMed] [Google Scholar]
- Silvapulle MJ. (1995). A Hotelling’ s T2-type statistic for testing against one-sided hypotheses. Journal of Multivariate Analysis, 55, 312–319. [Google Scholar]
- Sipes M, Matson JL, Worley JA, & Kozlowski AM (2011). Gender differences in symptoms of Autism Spectrum Disorders in toddlers. Research in Autism Spectrum Disorders, 5(4), 1465–1470. 10.1016/j.rasd.2011.02.007 [DOI] [Google Scholar]
- Stevens SS (1951). Mathematics, measurement, and psychophysics. [Google Scholar]
- Storch EA, Arnold EB, Lewin AB, Nadeau JM, Jones AM, De Nadai AS, … & Murphy TK (2013). The effect of cognitive-behavioral therapy versus treatment as usual for anxiety in children with autism spectrum disorders: A randomized controlled trial. Journal of the American Academy of Child & Adolescent Psychiatry, 52, 132–142. [DOI] [PubMed] [Google Scholar]
- Storch EA, Ehrenreich May J, Wood JJ, Jones AM, De Nadai AS, Lewin AB, … & Murphy TK (2012). Multiple informant agreement on the anxiety disorders interview schedule in youth with autism spectrum disorders. Journal of Child and Adolescent Psychopharmacology, 22, 292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch EA, Lewin AB, Collier AB, Arnold E, De Nadai AS, Dane BF, … & Murphy TK (2015). A randomized controlled trial of cognitive-behavioral therapy versus treatment as usual for adolescents with autism spectrum disorders and comorbid anxiety. Depression and anxiety, 32, 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch EA, Lewin AB, De Nadai AS, & Murphy TK (2010). Defining treatment response and remission in obsessive-compulsive disorder: a signal detection analysis of the Children's Yale-Brown Obsessive Compulsive Scale. Journal of the American Academy of Child & Adolescent Psychiatry, 49, 708–717. [DOI] [PubMed] [Google Scholar]
- Storch EA, Schneider SC, De Nadai AS, Selles RR, McBride NM, Grebe SC, …& Lewin AB (2019). A Pilot Study of Family-Based Exposure-Focused Treatment for Youth with Autism Spectrum Disorder and Anxiety. Child Psychiatry and Human Development. doi: 10.1007/s10578-019-00923-3 [DOI] [PubMed] [Google Scholar]
- Ung D, Wood JJ, Ehrenreich-May J, Arnold EB, Fuji C, Renno P, … & Storch EA (2013). Clinical characteristics of high-functioning youth with autism spectrum disorder and anxiety. Neuropsychiatry, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steensel FJ, Bögels SM, & Perrin S (2011). Anxiety disorders in children and adolescents with autistic spectrum disorders: a meta-analysis. Clinical Child and Family Psychology Review, 14, 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steensel FJA, & Heeman EJ (2017). Anxiety Levels in Children with Autism Spectrum Disorder: A Meta-Analysis. Journal of Child and Family Studies, 26(7), 1753–1767. doi: 10.1007/s10826-017-0687-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, Hoff A, Villabø MA, Peterman J, Kendall PC, Piacentini J, … & Sherrill J (2014). Assessing anxiety in youth with the multidimensional anxiety scale for children. Journal of Clinical Child & Adolescent Psychology, 43, 566–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JJ, & Drahota A (2005). Behavioral Interventions for Anxiety in Children with Autism. Los Angeles, CA: University of California–Los Angeles. [Google Scholar]
- Wood JJ, Drahota A, Sze K, Har K, Chiu A, & Langer DA (2009). Cognitive behavioral therapy for anxiety in children with autism spectrum disorders: A randomized, controlled trial. Journal of Child Psychology and Psychiatry, 50, 224–234.≠ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JJ, Kendall PC, Wood KS, Kerns CM, Seltzer M, Small BJ, Lewin AB, and Storch EA (2020). Cognitive behavioral treatments for anxiety in children with autism spectrum disorder a randomized clinical trial. JAMA Psychiatry, 77(5), 474–483. 10.1001/jamapsychiatry.2019.4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JJ, Ehrenreich-May J, Alessandri M, Fujii C, Renno P, Laugeson E, … & Murphy TK (2015). Cognitive behavioral therapy for early adolescents with autism spectrum disorders and clinical anxiety: A randomized, controlled trial. Behavior Therapy, 46, 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.