Abstract
Dentinal hypersensitivity (DH) is a common clinical condition usually associated with exposed dentinal surfaces. The aim of this study was to study the effect of sodium fluoride varnish, Gluma, and Er,Cr:YSGG laser, in the dentin hypersensitivity treatment. One hundred sixty-five teeth with dentin hypersensitivity in 55 patients were involved in this study. Teeth are divided into five groups based on the received treatment (n = 33): G group: Gluma; F group: sodium fluoride varnish (5%); L group: Er,Cr:YSGG laser (wavelength 2780 nm, frequency 20 Hz, power 0.25 W, energy density 44.3 J/cm2, and pulse width of 150 µs at distance of 1 mm for 30 s) which was followed by Er,Cr:YSGG laser; GL group: Gluma + laser; VL group: both sodium fluoride varnish and Gluma, which are common treatments for hypersensitivity, were selected as control groups. The treatment was performed in one session, and the sensitivity to air spray conditioning was recorded after the treatment, at 15 min, 1 week, 1 month, and 6 months as the VAS. Statistical analysis was performed using SPSS Ver. 21 software. One-way ANOVA was used to compare the VAS between all treatment groups at each time-point. One-way repeated measurements ANOVA (RM-ANOVA) and two-way-repeated measurements ANOVA (RM-ANOVA) were used to compare the hypersensitivity of each group and sensitivity of all treatment groups, respectively. Tukey post hoc test was used to compare the groups pairwise. The hypersensitivity between different groups at before and 15 min after the treatment was not significantly different (P = 0.063). The hypersensitivity of all studied groups was decreased after the treatment. The Er,Cr:YSGG laser, alone or in combination with Gluma, in 1 week, 1 month, and 6 month follow-ups, had significantly reduced the hypersensitivity instead of sodium fluoride varnish. All treatments significantly reduced the dentin hypersensitivity up to 6 months. Er,Cr:YSGG laser alone or in combination with Gluma was more effective than sodium fluoride varnish; however, it was not significantly different from other treatments. In a 6-month follow-up of dentine hypersensitivity treatment, Gluma had a significantly higher effect than sodium fluoride. Trial registration: IRCT20190422043343N1. Registered 19 July 2019.
Keywords: Dentinal tubule, Desensitizer agent, VAS score
Background
Dentin hypersensitivity is a painful response to sensory stimuli that usually does not occur in healthy and normal teeth. The main dentin hypersensitivity characteristic is short sharp pain arising in the exposed dentin in response to stimuli and cannot be ascribed to any other diseases, dental defects, or restorative treatments and pathology in the tooth. This stimulus can be thermal, evaporative, osmotic, static, or chemical [1]. Dentin hypersensitivity as a painful condition affects approximately 20% of the population [2]. It is sometimes difficult to diagnose this disease, because other possible causes of the pain must first be evaluated. All dentists should be familiar with dentin hypersensitivity to relieve the patient’s pain effectively, especially in severe and debilitating cases [3].
Therapeutic strategies to reduce the dentin hypersensitivity are from prescribing desensitizer agents at home in mild cases, treatment in office in cases of no improvement or in severe, root canal treatment in very severe cases not responding to any at home or in office methods that only one tooth shows symptoms [4]. Various desensitizer agents with different mechanisms such as ProArgin™ (arginine 8%) and novamin (calcium phosphosilicate 5%) [5], nanohydroxyapatite 25% [6], and concentrations of 5 to 10% potassium oxalate [7] have been used in the dentin hypersensitivity treatment. Fluoride varnish closes the dentinal tubules by forming a mechanical barrier. The effect of fluoride to reduce the dentine hypersensitivity has been proven as well [8, 9]. Gluma as a commercial desensitizer agent containing an aqueous hydroxyethylene methacrylate solution (35%) and glutarldehyde (5%) is effective in dentine hypersensitivity treatment. Dentinal tubules are blocked with glutaraldehyde, which neutralizes the hydrodynamic mechanism of tubules and reduces the hypersensitivity [9, 10]. Significant effect of this agent in dentine hypersensitivity treatment has been shown up to 6 months after its application [11–14].
Today, the use of different types of lasers in dentistry has been extensively studied [12, 15]. The use of laser has been shown to be effective in dentine hypersensitivity reducing, both in short and long term [16]. A systematic review study showed that all four laser types as Er,Cr:YSGG, Nd:YAG, Er:YAG, and GaAlAs have significant effects on dentine hypersensitivity reducing immediately and in a long-term treatment [17]. Recent studies have shown satisfactory results in dentine hypersensitivity treatment with laser after 3 and 6 months, although studies have recommended additional clinical trials to compare the effectiveness of these therapies in a long-term treatment [18]. Nd:YAG, Er,Cr:YSGG, and CO2 lasers, due to their ability to melt the pre-tubular dentine, can partially or completely block the dentinal tubules and reduce dentine hypersensitivity [19]. An SEM evaluation showed a decrease in the dentinal tubules’ diameter and consequently a decrease in dentine hypersensitivity following Nd:YAP laser irradiation [20]. In addition to be effective in dentine hypersensitivity treatment, laser can be combined with various desensitizer agents such as potassium oxalate, sodium, and calcium fluoride, ProArgin™, nano carbonate apatite, Gluma, bioglass, nano fluorohydroxyapatite, toothpaste, and desensitizer mouthwash which reduce the dentine hypersensitivity [10, 21–24].
Given the painful nature of dentine hypersensitivity and lack of adequate evidence for the most effective treatment which eliminate the pain completely and permanently, in this study the effects of two conventional therapies, Gluma desensitizer and sodium fluoride varnish (5%), with or without Er,Cr:YSGG laser irradiation were studied to evaluate the combination of treatments with laser irradiation on the dentine hypersensitivity treatment. The study hypothesis is that combination of existing standard treatments with laser irradiation can increase their effectiveness on the dentine hypersensitivity treatment. A 6-month follow-up of patients in this study shows how long the effects of each method can maintain.
Methods
Ethical perspectives
This randomized double-blind clinical trials study was performed on 55 eligible patients who were referred to the Restorative Dentistry Department of Hamadan University of Medical Sciences during Aug to Oct 2019 for dentin hypersensitivity treatment. The study received approval from the Ethics Committee of Hamadan University of Medical Sciences (IR.UMSHA.REC.1398.095) and was registered in the Iranian Registry of Clinical Trials (IRCT20190422043343N1). Written consent was obtained from all participants.
Sample size calculation
The significant level and test power were 95% and 80%, respectively. The expected difference in the mean sensitivity was and the standard deviation was . Sample size was calculated to be 30. Due to the possibility of cancelation or loss of samples, 3 teeth were added to each group and finally, in each group, 33 teeth were examined (165 samples in 5 groups).
Inclusion and exclusion criteria
Participants included in the study had the following conditions:
Good general health without any known allergy to commercial dental products. 2. The teeth are free of decay or restoration.
Patients have not previously received any dentine hypersensitivity treatments.
Patients have no history of receiving any teeth whitening treatments.
The degree of tooth initial sensitivity should be 3 or higher based on VAS.
Participants excluded from the study had the following conditions:
Patients with chronic diseases and daily pain episodes who have taken anticonvulsants, analgesics, antihistamines, sedatives, anti-inflammatories, or painkillers in the past 72 h.
Patients who have used desensitizing toothpaste or mouthwash in the last 3 months.
Patients who have had periodontal surgery in the last 6 months.
Vital or non-vital teeth that have cracks, previous restoration, carious lesions, gingival recession of more than 3 mm or active periodontal disease.
Teeth with deep carious lesions in the vicinity of sensitive teeth that their pain can be confused with tooth sensitivity.
Patients whose sensitive teeth are adjacent together.
Patients were informed about the aim of study and its design, and informed consent was obtained. The relevant forms were approved by the Ethics Committee of Hamadan University of Medical Sciences.
Randomization and blinding
For randomization, the teeth were first classified into two groups according to the initial sensitivity to an air-syring flow with a pressure of 45 psi and air temperature of 21–22 °C as low sensitive (≤ 5 in VAS) and high sensitive (5 > in VAS) by block randomization method [25]. Block size and randomization method were performed by someone other than treatment and sensitivity assessment implementers. Finally, all 165 teeth were randomly divided into 5 groups (n = 33).
To blind the study, one operator performed laser irradiation and applied the sodium fluoride and Gluma to the teeth surface, while another operator, who was unaware of the patient’s treatment protocol, assessed the teeth sensitivity. Both operators were trained and calibrated individuals who had passed the accuracy tests. Patients were also unaware of the received treatment. The patients, who’s their treatment protocol did not include actual laser photonic energy, but rather received aiming beam irradiation at distance of 1 mm as a sham treatment.
Clinical process
At the beginning and end of study, the vitality of each tooth that was to be treated was evaluated by Endo-Frost cold spray (Coltene Whaledent, Germany) which was applied to the buccal surface of each tooth for 10 s and it was confirmed through a cold feeling by patient. All patients were advised not to receive any other treatment at the same time as participating in the study. One week before the study, teeth of all patients received phase 1 periodontal treatment as scaling and root planing (SRP) and oral hygiene instructions. During the study, participants used a soft toothbrush and a free desensitizer agent toothpaste as fluoride or etc. (fluoride-free Colgate toothpaste, Colgate, USA). All patients were advised to avoid brushing with excessive force and excess consumption of sour or acidic food. Prior of treatment, the degree of tooth sensitivity to evaporative stimuli was evaluated by an air-syring flow with a pressure of 45 psi and air temperature of 21–22 °C [26]. In each session, before working, the pressure was controlled with a barometer. The tooth was dried and isolated, and a stream of cold air was inserted into the buccal surface of the tooth for 35 s from a distance of 2 mm, and the tooth’s response to this stimulus was recorded via visual analog scale (VAS). The VAS score is a score that patient gives in response to a painful stimulus. The lowest score was zero which means a completely painless situation, and the highest score was 10 which means the most painful thing that a human can experience. Any number reported by the patient was considered degree of sensitivity. After recording the tooth initial sensitivity, according to the group that was randomly assigned, the tooth received one of the following treatment protocols:
L group
The tooth was dried with air-syring flow with a pressure of 45 psi and isolated with a cotton roll. Er,Cr:YSGG laser (i plus, Biolase, Inc., USA) was applied perpendicular to the buccal surface in Smode by scanning movement defocusing at distance of 1 mm with water (0%) and air (0%) for 30 s. Laser setting was as follows: wavelength of 2780 nm, frequency of 20 Hz, power of 0.25 W, energy density of 44.3 J/cm2, and pulse width of 150 µs, with M Z6 sapphire gold handpiece and 600 microns diameter of the laser tip and 6 mm length tip [27].
V group
The tooth was dried and isolated like first group to prevent the varnish from mixing with saliva. Two thin layers of sodium fluoride varnish 5% (Duraphat®, Colgate Oral Pharmaceuticals, New York, USA) were rubbed on the tooth surface with a disposable micro-brush according to the factory’s instructions. Patients are instructed to refuse from eating any carbonated foods or beverages for 1 h after the varnish application. The cotton roll was removed to allow moisture to set the varnish. Aiming beam was applied to the tooth surface for 30 s.
VL group
The varnish was used on the tooth surface according to the method used in V group. The varnish remained for 60 s and cotton roll was reinserted. The laser was applied to the tooth surface with the same irradiation characteristics of L group.
G group
After drying tooth surface and isolation with cotton roll same as other groups, the Gluma (Heraeus Kulzer GmbH, Hanau, Germany) was applied with rubbing motion gently but firm. After 30 to 60 s, the dentine was completely dried until the liquid disappears and the surface entirely loses its gloss. Aiming beam was applied to the tooth surface for 30 s.
GL group
Gluma was used on the tooth surface similar to the method used in G group. The laser, with the same irradiation characteristics of L group, was applied to the tooth surface.
In this study, laser irradiation was performed based on the safety protocols and international standards. All groups and the therapist used safety glasses. Sodium fluoride varnish and Gluma, which are common treatments for hypersensitivity, were both selected as control groups.
Recall sessions and dentine hypersensitivity evaluation
After treatment, the air syringe sensitivity was recorded at 15 min, 1 week, 1 month, and 6 months after the treatment. All assessments were performed by an operator. Oral hygiene instructions were given to the patients at each visit, but no prophylactic treatment was done until the end of study. At this session, subjective symptoms such as allergic reactions, burning sensation, sores and changes in taste, as well as objective symptoms such as redness of oral mucosa and teeth discoloration were evaluated.
Statistical analysis
Statistical analyses were performed using SPSS Ver. 21 software. One-sample Kolmogorov–Smirnov test was used to evaluate the normal distribution of the data. One-way ANOVA was used to compare the VAS between treatment groups at each time-point. Tukey post hoc test was used to compare the groups pairwise. One-way repeated measurement ANOVA (RM-ANOVA) was used to compare the sensitivity of each group among the study, and two-way repeated measurements ANOVA (RM-ANOVA) was used to compare the sensitivity of treatment groups. A Bonferroni post hoc test was used to compare the treatment follow-up times pairwise. Significant level was considered as P ≤ 0.05.
Results
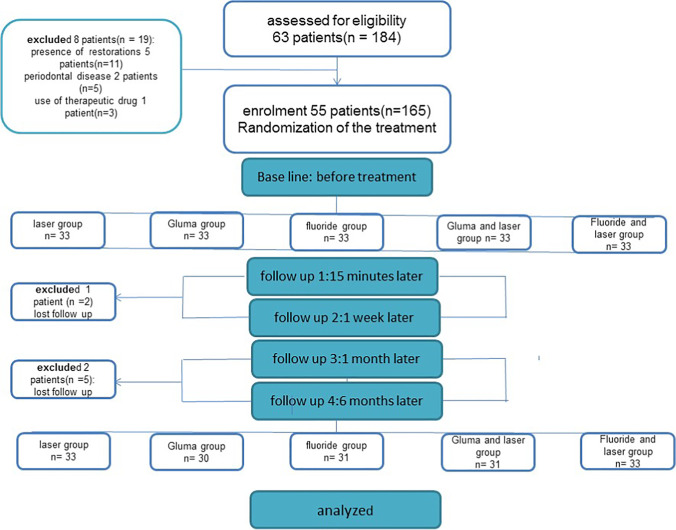
From 55 patients, one patient did not complete the follow-up session due to the migration and two patients did not complete the follow-up session due to the coronavirus pandemic, and finally 52 patients with 158 sensitive teeth (33 teeth in L group, 31 teeth in F group, 33 teeth in LF group, 30 teeth in G group, and 31 teeth in GL group) involved in the study (Fig. 1). The study participants included 23 males and 32 females with mean age of 36 years old. No allergy reactions, burning sensations, sores and changes in taste, mucosa redness, and teeth discoloration were observed in any of patients. Data normality was assessed. Homogeneity of groups, in terms of initial sensitivity, was confirmed by chi-square test (P = 0.260). Comparing between sensitivity in each treatment group at different interval times is presented in Table 1. The results of RM-ANOVA test showed that there was a statistically significant difference between study groups during the follow-up time (P = 0.034). The sensitivity change during the time is also statistically significant (P ≤ 0.001) (Table 1). One-way ANOVA test showed that the sensitivity in different groups at beginning of study was not statistically significant. Fifteen minutes after the treatment, the difference in sensitivity between groups was not statistically significant, but in 1 week, 1 month, and 6 month follow-ups, there was a statistically significant difference between studied groups (Table 1). The lowest sensitivity at 15 min, 1 week, 1 month, and 6 months was shown in L and LG groups, and the highest sensitivity was shown in F group. The results of Tukey test showed that at 1 week, 1 month, and 6 months after the treatment, the differences between L and F groups and LG and F groups were statistically significant (P ≤ 0.05) (Table 2).
Fig. 1.
The initial and final study participants
Table 1.
Comparing between sensitivity in each treatment group at different interval times
| S0 mean ± SD median (IQR) |
S15 mean ± SD median (IQR) |
SW mean ± SD median (IQR) |
S1M mean ± SD median (IQR) |
S6M mean ± SD median (IQR) |
P-value/intragroup comparison | |
|---|---|---|---|---|---|---|
| G |
5.26 ± 2.09 5.00 (3.25) |
2.80 ± 1.66 3.00 (1.50) |
2.56 ± 1.69 2.00 (3.00) |
2.56 ± 1.61 2.00 (3.00) |
2.43 ± 1.77 2.50 (3.00) |
≤ 0.001 |
| L |
5.87 ± 2.05 6.00 (4.00) |
1.81 ± 1.97 2.00 (3.00) |
1.63 ± 2.07 1.00 (2.50) |
1.90 ± 1.82 2.00 (3.00) |
2.24 ± 1.88 2.00 (3.00) |
≤ 0.001 |
| F |
5.29 ± 1.41 6.00 (2.00) |
2.87 ± 2.10 2.00 (3.00) |
3.38 ± 2.04 3.00 (3.00) |
3.51 ± 2.43 3.00 (3.00) |
4.25 ± 2.51 5.00 (4.00) |
≤ 0.001 |
| LG |
5.83 ± 1.79 6.00 (2.00) |
1.80 ± 2.12 1.00 (2.00) |
1.51 ± 2.35 0.00 (2.00) |
1.96 ± 2.02 2.00 (3.00) |
2.32 ± 2.27 2.00 (3.00) |
≤ 0.001 |
| LF |
6.12 ± 1.81 6.00 (3.00) |
2.15 ± 1.64 2.00 (2.00) |
2.36 ± 1.81 2.00 (3.50) |
2.51 ± 1.60 2.00 (3.00) |
3.06 ± 1.80 3.00 (2.00) |
≤ 0.001 |
| P-value/intergroup comparison | 0.260 | 0.063 | 0.002 | 0.008 | 0.001 |
G, Gluma; L, laser; F, fluoride; LG, Gluma + laser; LF, fluoride + laser; S0, degree of sensitivity before treatment; S15, degree of sensitivity at 15 min after treatment; SW, degree of sensitivity at 1 week after treatment; S1M, degree of sensitivity at 1 month after treatment; S6M, degree of sensitivity at 6 months after treatment
Table 2.
Intergroup comparison between two treatments at different times
| SW | S1M | S6M | Overall | |
|---|---|---|---|---|
| G-L | 0.357 | 0.656 | 0.996 | 0.305 |
| G-F | 0.503 | 0.306 | 0.007 | 0.084 |
| G-LG | 0.251 | 0.742 | 1.000 | 0.305 |
| G-LF | 0.995 | 1.000 | 0.750 | 0.782 |
| L-F | 0.006 | 0.009 | 0.001 | 0.005 |
| L-LG | 0.999 | 1.000 | 1.000 | 0.987 |
| L-LF | 0.583 | 0.703 | 0.495 | 0.183 |
| F-LG | 0.003 | 0.016 | 0.003 | 0.006 |
| F-LF | 0.254 | 0.233 | 0.146 | 0.135 |
| LG-LF | 0.445 | 0.786 | 0.611 | 0.184 |
The results of intragroup comparison showed that the trend of sensitivity changes was statistically significant for all studied groups during the time (P ≤ 0.001). For all groups, changes in the follow-up times (15 min, 1 week, 1 month, 6 months) were statistically significant (P ≤ 0.001). In F group, the sensitivity has reduced till 15 min while it was increased at other follow-up times. In G group, the sensitivity changes after the treatment have decreased (Table 3).
Table 3.
Intragroup comparison pairwise method—for each treatment
| G | L | F | LG | LF | |
|---|---|---|---|---|---|
| S0-S15 | ≤ 0.001 | ≤ 0.001 | ≤ 0.001 | ≤ 0.001 | ≤ 0.001 |
| S0-SW | ≤ 0.001 | ≤ 0.001 | ≤ 0.001 | ≤ 0.001 | ≤ 0.001 |
| S0-S1M | ≤ 0.001 | ≤ 0.001 | ≤ 0.001 | ≤ 0.001 | ≤ 0.001 |
| S0-S6M | ≤ 0.001 | ≤ 0.001 | 0.002 | ≤ 0.001 | ≤ 0.001 |
| S15-SW | 0.269 | 0.325 | 0.177 | 0.059 | 0.315 |
| S15-S1M | 0.293 | 0.756 | 0.031 | 0.524 | 0.116 |
| S15-S6M | 0.360 | 0.228 | ≤ 0.001 | 0.040 | ≤ 0.001 |
| SW-S1M | 1.000 | 0.414 | 0.704 | 0.138 | 0.304 |
| SW-S6M | 0.728 | 0.127 | 0.028 | 0.004 | 0.003 |
| S1M-S6M | 0.631 | 0.039 | 0.013 | 0.094 | 0.005 |
Discussion
Today, there are several treatments for dentine hypersensitivity, including a wide range of at-home and in-office methods. It was recommended to start treatment with less invasive at-home methods, and in case the pain persists, professional and aggressive methods need to be used [28]. It has been shown that dentine hypersensitivity treatment improves the patient’s quality of life [29, 30].
In this study, different treatment methods were used including Gluma and sodium fluoride varnish (both as control groups), Er,Cr:YSGG laser, Er,Cr:YSGG laser + Gluma combination, and Er,Cr:YSGG laser + fluoride combination. Based on the results of this study, it can be concluded that all the used methods are suitable for dental hypersensitivity reducing in patients, and depending on the conditions, dentists can use any of these methods for the treatment. It is difficult to quantify the patient’s dentine hypersensitivity due to the subjective nature of pain. At this time, several methods have been used to quantify the hypersensitivity degree, among which the use of visual analog scale (VAS) is of great interest [31].
As a desensitizer agent, Gluma contains glutaraldehyde, improves coagulation in the dentinal tubules, and blocks them. Gluma blocks the dentinal tubules by two reactions. First, glutaraldehyde reacts with serum albumin in the tubular fluid, which induces the serum albumin deposition, and second, the glutaraldehyde reaction with serum albumin induces HEMA polymerization. Following this reaction, the dentinal tubules are blocked to a depth of 200 microns, which helps to reduce the patient’s pain [32]. The positive effects of this agent in dentine hypersensitivity reducing have been reported [5, 9–11, 33], although some studies are opposed to it [34, 35]. Mehta et al. compared Gluma with three desensitizer agents (Nanoseal, MS Coat One F, and Teethmate Desensitizer). Gluma significantly retains its desensitizing effects for 6 months, and VAS score for immediate treatment and 6 months after treatment did not shown significant difference which is in agreement with the results of this study [36]. Ahmed et al. compared the effect of Gluma (GLUMA® Comfort® Bond + Desensitizer) with sodium fluoride varnish at 30 days after the treatment. They showed that Gluma was more effective in reducing the dentine hypersensitivity compared to sodium fluoride varnish. In the present study, Gluma and sodium fluoride were not significantly different at 1-month follow-up, but it showed significantly better performance than sodium fluoride in a 6-month follow-up [37]. Aranha et al. compared Gluma, seal and protect (SP), oxagel, sodium fluoride, and low-level lasers in 1-week and 1-, 3-, and 6-month follow-ups. They showed that all of these methods, despite the different mechanisms of action, were effective in reducing the dentine hypersensitivity at 6 months compared to the baseline. But only Gluma and SP had immediate results [38]. In all studies in which Gluma retained its 6-month effects, such as our study, prior to the treatment, patients were given recommendations on diet, type of toothbrush and toothpaste, and how to brush properly, and in the follow-up sessions, the patient was reminded because the effects of these agents are expected to gradually decrease with the wear caused by tooth brushing and foods.
Fluoride reduces the dentine hypersensitivity via precipitation of calcium fluoride into the dentinal tubules. Significant effect of this agent in the dentine hypersensitivity reducing has been demonstrated up to 90 days by Nardi et al. [8]. In the present study, the effect of sodium fluoride varnish (5%) up to 6 months after treatment was confirmed, although this effect began to increase from 15 min after treatment. Also, at 6-month follow-up, the effect of this agent was significantly less than laser, Gluma or their combination. This confirms the hypothesis that laser and its combinations are more effective than conventional treatments. In another study, the effect of fluoride varnish and Sylc (a bioactive glass-based powder) delivered by the air polishing system in dentine hypersensitivity reducing over 3 min; 1, 2, 3, and 4 weeks; and 6 months and 1 year was studied. The obtained results showed that Sylc significantly reduced the sensitivity over 3 weeks to a year, while the effects of sodium fluoride were limited to the first week of use. The short-term effect of sodium fluoride is related to its needs for several periods of use. It helps fluoride adhesion to the tooth surface causing a long-term effect. However, a single period of its use cannot have the long-term effects [39]. On the contrary, Femiano et al. showed that several use of diode laser, sodium fluoride, and Gluma did not considerably improve the treatment efficacy. Sodium fluoride alone performed a lower effect in order to both 1- and 6-month follow-ups than diode laser and laser with sodium fluoride and Gluma. In continuation, they related the weak effect of sodium fluoride in short and long term to the rapid dissolution in saliva causing loss of CaF2 layer which blocks tubules [21]. These results suggest combining the sodium fluoride with methods such as laser to reduce its solubility. In this study, Er,Cr:YSGG laser was used. The use of lasers may be useful in two ways. First, the thermal energy provided by laser irradiation increases the mobility of the desensitizer molecules, which helps them to better penetrate the tubule. Second, an important feature of Er,Cr:YSGG laser is its high adsorption in water and hydroxyapatite and evaporating dentine fluid from exposed tubules into the oral environment and leaving insoluble salts, which block dentinal tubules and reduce the dentine hypersensitivity [18]. A systematic review in 2020 comparing the effect of lasers and desensitizers showed that there is no strong evidence that these methods are superior to each other, and emphasizes the need of further clinical trials [40]. Pourshahidi et al. compared the effect of diode laser irradiation (940 nm) for 1 min and Er,Cr:YSGG laser irradiation with a wavelength of 2780 nm and a pulse width of 140–200 μm on sensitive teeth and at least for short term it shows Er,Cr:YSGG superiority to diode laser. So, we used Er,Cr:YSGG laser in our study [26].
Comparing 0.25 and 0.5 W powers of Er,Cr:YSGG laser, Yilmaz et al. showed that both powers immediately reduced the dentine hypersensitivity, but this effect was better in the 0.5 W group. They measured VAS only immediately after treatment and did not evaluate their long-term effects. Our study shows that 0.25 power results are acceptable even for up to 6 months so to reduce the risk of injury of the pulp, there is no need to increase the power [25]. Chaudhry et al. showed that the efficacy of Er,Cr:YSGG of 0.25 W laser was more significant than Gluma desensitizer, SP, sealants, and sodium fluoride varnish immediately and after 2 months [9]. In our study, the combination of desensitizer agents and laser was also used and showed that although the use of lasers with Gluma and sodium fluoride can improve their effectiveness compared to the single use at all follow-up times, this improvement is not significantly effective which may be due to the fact that the laser was applied after Gluma and sodium fluoride and did not intensify their effects. Perhaps if the laser irradiation was the first treatment part, the microscopic changes in dentin surface improve their effects. However, laser irradiation parameters need to be optimized to obtain the safe and effective irradiation. The presence of chemicals on the dentinal tubules may absorb the energy of irradiated laser photons (high absorption of laser in the water in the base gel and its evaporation reduces the effect of laser), so we recommend a study in which laser is applied before gels and after narrowing the dentin tubules based on the local thermal mechanism of the laser, increase or decrease in the effects of these gels is evaluated. However, an in vitro study is recommended to evaluate its quantitative and qualitative effects with electron microscopy and other methods. Other studies have shown that the combination of physical and chemical therapies to reduce the dentine hypersensitivity can be attributed to the use of different sensitizers, different lasers, and different laser settings. This improvement can be achieved through a variety of mechanisms. For example, laser energy can improve the release of desensitizer agents into the tubules or block the tubules to prevent the loss of these materials.
Hsu et al. showed that fluoridated dentinal tubule-occluding agents (FDTOA) formed fine crystalline deposits on the dentine surfaces. By immersing dentinal samples into vitamin C (0.5 M) for 3 h or brushing 3600 times with an electric toothbrush, these crystalline deposits were partially or completely removed from the dentine surfaces. When dentin samples, in addition to FDTOA compounds, irradiated with Nd-YAG laser, laser melts and re-crystallizes the dentine surface, and the dentine blocking material is trapped inside the dentinal tubules. So immersing in vitamin C and brushing could not remove FDTOA from inside of tubules, and the combination of fluoride compounds with Nd-YAG laser increases their effectiveness in blocking dentinal tubules [22]. Kumar et al. reported the same results on the combination of sodium fluoride varnish (5%) and Nd-YAG laser [41]. Ozlem et al. compared the effect of Gluma, Nd:YAG laser (1 W/cm2, 10 Hz) and Er,Cr:YSGG laser (0.25 W/cm2, 20 Hz) and combination of Gluma with each of these lasers. For 30 min, and 7–90 and 180 days, they found that dentine hypersensitivity decreased significantly in all studied groups after the treatment session. The Er,Cr:YSGG laser was most effective in hypersensitivity treatment with or without Gluma. The results of Ozlem et al. are consistent with our results.
In an in vitro study, SEM micrographs of five treatment groups (control group, Colgate desensitizer toothpaste, MI pastes desensitizer pasteVivaSens fluoride desensitizer, Er,Cr:YSGG laser) showed that, immediately after treatment, most dentinal tubules were completely obliterated. After 2 weeks, only stable blocked tubules were observed in the toothpaste group and Er,Cr:YSGG laser group, and finally, after 1 month, only in Er,Cr:YSGG laser group, the dentinal tubules were completely blocked or at least narrowed. Therefore, a stable treatment of dentine hypersensitivity, during a month, is obtained with Er,Cr:YSGG laser, which confirms our results that the effect of sensitivity reducing by using laser continues for up to 1 week and 1 month without significant change, and then gradually decreased its effectiveness [42].
Another in vitro study comparing the number of blocked dentinal tubules per 100 mm2 of dentine after using Gluma, Teethmate, Nd:YAG laser, Er:YAG laser, and Er:YAG laser in combination with these two agents showed that laser and laser in combination with desensitizers have the highest blockage of dentinal tubules. This study also showed that Gluma with Er:YAG laser had significantly a higher number of blocked tubules than other groups [23]. However, in our study, although the use of lasers with Gluma and sodium fluoride has improved the effectiveness of these agents, this improvement has not been statistically significant and same results can be achieved with using of each method alone. The fact that combination therapy is effective in some studies and useless in others may be related to using different laser settings as radiation parameters and laser irradiation time as before or after the desensitizer application.
There are several factors that contribute the improvement and progression of dentine hypersensitivity. Tooth brush types, brushing methods, heavy dental contacts that cause tooth to bend, acidic foods and beverages, and carbonated beverages, etc. cannot be fully controlled only by training and advising patients. On the other hand, a part of the sensitivity reducing in each patient may be due to the spontaneous relief of pain due to the body reactions. Because a negative control group without receiving any treatment was not included in our study, the percentage of spontaneously relieved teeth was not determined. As this study was clinical, all participants had sensitivity scores above 3 who came to us for treatment and not treating them was not morally correct, so it was not possible to have a negative control group and this was a limitation of the study. In this study, all treatment methods were performed in just one session. Further studies are recommended with more treatment sessions to determine whether repeat therapy can improve the results or not. It is also recommended that these methods be compared with new methods such as the use of Nd:YAP lasers and nanohydroxyapatites [20].
Conclusion
The results of this study showed that Gluma, Er,Cr:YSGG laser, sodium fluoride varnish, laser and Gluma combination, and laser and sodium fluoride varnish combination treatment methods significantly reduce the dentine hypersensitivity for up to 6 months. In 1-week, 1-month, and 6-month follow-ups, and generally in laser or in laser combination with Gluma, the treatment is significantly more effective than sodium fluoride but not significantly different from other groups. In 6-month follow-up, Gluma had a significantly higher effect than sodium fluoride.
Abbreviations
- DH
Dentinal hypersensitivity
- RM-ANOVA
Repeated measurements ANOVA
- SRP
Scaling and root planing
- VAS
Visual analog scale
- SP
Seal and protect
Author contribution
MF1 and LR designed the study. MF1 carried out all data collection. AF, MF1, EY, LR, RF, and MGK edited and revised the manuscript. MF2 was responsible for the statistical analyses. All authors read and approved the final manuscript.
Funding
This study was financially supported by a grant (9805153779) from the Research Deputy of Hamadan University of Medical Sciences.
Data availability
All supporting data are included as additional files, upon request.
Declarations
Ethics approval and consent to participate
The study design and all patient forms, including consent form, received ethics approval from the ethics committee of the Hamadan University of Medical Sciences (IR.UMSHA.REC.1398.095).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mahsa Forouzande, Email: mahsaforouzande@gmail.com.
Abbas Farmany, Email: a.farmany@usa.com.
References
- 1.Suri I, Singh P, Shakir QJ, Shetty A, Bapat R, Thakur R. A comparative evaluation to assess the efficacy of 5% sodium fluoride varnish and diode laser and their combined application in the treatment of dentin hypersensitivity. J Indian Soc Periodontol. 2016;20(3):301–307. doi: 10.4103/0972-124X.181243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal S. Dentin hypersensitivity: etiology, symptoms, diagnosis and recent trends in management. Int J Clin Prev Dent. 2019;15(2):73–76. doi: 10.15236/ijcpd.2019.15.2.73. [DOI] [Google Scholar]
- 3.Goh V, Corbet EF, Leung WK. Impact of dentine hypersensitivity on oral health-related quality of life in individuals receiving supportive periodontal care. J Clin Periodontol. 2016;43(7):595–602. doi: 10.1111/jcpe.12552. [DOI] [PubMed] [Google Scholar]
- 4.Miglani S, Aggarwal V, Ahuja B. Dentin hypersensitivity: recent trends in management. J Conserv Dent. 2010;13(4):218–224. doi: 10.4103/0972-0707.73385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samuel S, Khatri S, Acharya S, Patil S. Evaluation of instant desensitization after a single topical application over 30 days: a randomized trial. Aust Dent J. 2015;60(3):336–342. doi: 10.1111/adj.12341. [DOI] [PubMed] [Google Scholar]
- 6.Ameen S, Niazy M, El-yassaky M, Jamil W, Attia M. Clinical evaluation of nano-hydroxyapatite as dentin desensitizer. Al-Azhar Dent J Girls. 2018;5(1):79–87. doi: 10.21608/adjg.2018.7995. [DOI] [Google Scholar]
- 7.da Mata GA, Zeola LF, Moura GF, Teixeira DNR, de Queiroz Gonzaga RC, da Silva GR, et al. A long-term evaluation of experimental potassium oxalate concentrations on dentin hypersensitivity reduction: a triple-blind randomized clinical trial. J Dent. 2019;89:103180. doi: 10.1016/j.jdent.2019.103180. [DOI] [PubMed] [Google Scholar]
- 8.Nardi G, Sabatini S, Lauritano D, Silvestre F, Petruzzi M. Effectiveness of two different desensitizing varnishes in reducing tooth sensitivity: a randomized double-blind clinical trial. ORAL Implantol. 2016;9(4):185. doi: 10.11138/orl/2016.9.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhry S, Yadav S, Talwar S, Verma M. A comparative evaluation of erbium, chromium: Yttrium-Scandium-Gallium-Garnet laser with three other desensitizing agents for the management of dentinal hypersensitivity: a hospital-based study. J Dent Lasers. 2018;12(1):18. doi: 10.4103/jdl.jdl_5_18. [DOI] [Google Scholar]
- 10.Dilsiz A, Aydın T, Emrem G. Effects of the combined desensitizing dentifrice and diode laser therapy in the treatment of desensitization of teeth with gingival recession. Photomed Laser Surg. 2010;28(S2):S-69–S-74. doi: 10.1089/pho.2009.2640. [DOI] [PubMed] [Google Scholar]
- 11.Lopes AO, Aranha ACC. Comparative evaluation of the effects of Nd: YAG laser and a desensitizer agent on the treatment of dentin hypersensitivity: a clinical study. Photomed Laser Surg. 2013;31(3):132–138. doi: 10.1089/pho.2012.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abduljabbar T, Vohra F, Akram Z, Ab Ghani SM, Al-Hamoudi N, Javed F. Efficacy of surgical laser therapy in the management of oral pigmented lesions: a systematic review. J Photochem Photobiol B Biol. 2017;173:353–359. doi: 10.1016/j.jphotobiol.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Yilmaz HG, Albaba MR, Caygur A, Cengiz E, Boke-Karacaoglu F, Tumer H. Treatment of recurrent aphthous stomatitis with Er, Cr: YSGG laser irradiation: a randomized controlled split mouth clinical study. J Photochem Photobiol B Biol. 2017;170:1–5. doi: 10.1016/j.jphotobiol.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 14.dos Santos SC, Oliveira HFF, de Souza Batista VE, Lemos CAA, Verri FR. Influence of low-level laser therapy on the healing of human bone maxillofacial defects: a systematic review. J Photochem Photobiol B Biol. 2017;169:83–89. doi: 10.1016/j.jphotobiol.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Borzabadi-Farahani A. Effect of low-level laser irradiation on proliferation of human dental mesenchymal stem cells; a systemic review. J Photochem Photobiol B Biol. 2016;162:577–582. doi: 10.1016/j.jphotobiol.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 16.Hu M-L, Zheng G, Han J-M, Yang M, Zhang Y-D, Lin H. Effect of lasers on dentine hypersensitivity: evidence from a meta-analysis. J Evid Based Dent Pract. 2019;19(2):115–130. doi: 10.1016/j.jebdp.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Kong Y, Lei Y, Li S, Zhang Y, Han J, Hu M. Network meta-analysis of the desensitizing effects of lasers in patients with dentine hypersensitivity. Clin Oral Investig. 2020;24(6):1917–1928. doi: 10.1007/s00784-019-03051-3. [DOI] [PubMed] [Google Scholar]
- 18.de Oliveira da Rosa WL, Lund RG, Piva E, da Silva AF. The effectiveness of current dentin desensitizing agents used to treat dental hypersensitivity: a systematic review. Quintessence Int. 2013;44(7):535–546. doi: 10.3290/j.qi.a29610. [DOI] [PubMed] [Google Scholar]
- 19.Gholami GA, Fekrazad R, Esmaiel-Nejad A, Kalhori KA. An evaluation of the occluding effects of Er; Cr: YSGG, Nd: YAG, CO2 and diode lasers on dentinal tubules: a scanning electron microscope in vitro study. Photomed Laser Surg. 2011;29(2):115–121. doi: 10.1089/pho.2009.2628. [DOI] [PubMed] [Google Scholar]
- 20.Fornaini C, Brulat-Bouchard N, Medioni E, Zhang S, Rocca J-P, Merigo E. Nd: YAP laser in the treatment of dentinal hypersensitivity: an ex vivo study. J Photochem Photobiol B Biol. 2020;203:111740. doi: 10.1016/j.jphotobiol.2019.111740. [DOI] [PubMed] [Google Scholar]
- 21.Femiano F, Femiano R, Lanza A, Festa MV, Rullo R, Perillo L. Efficacy of diode laser in association to sodium fluoride vs Gluma desensitizer on treatment of cervical dentin hypersensitivity. A double blind controlled trial. Am J Dent. 2013;26(4):214–8. [PubMed] [Google Scholar]
- 22.Hsu P-J, Chen J-H, Chuang F-H, Roan R-T. The combined occluding effects of fluoride-containing dentin desensitizer and Nd-Yag laser irradiation on human dentinal tubules: an in vitro study. Kaohsiung J Med Sci. 2006;22(1):24–29. doi: 10.1016/S1607-551X(09)70216-5. [DOI] [PubMed] [Google Scholar]
- 23.Öncü E, Karabekiroğlu S, Ünlü N. Effects of different desensitizers and lasers on dentine tubules: an in-vitro analysis. Microsc Res Tech. 2017;80(7):737–744. doi: 10.1002/jemt.22859. [DOI] [PubMed] [Google Scholar]
- 24.Solati M, Fekrazad R, Vahdatinia F, et al. Dentinal tubule blockage using nanobioglass in the presence of diode (980 nm) and Nd:YAG lasers: an in vitro study. Clin Oral Invest. 2022;26:2975–2981. doi: 10.1007/s00784-021-04279-8. [DOI] [PubMed] [Google Scholar]
- 25.Yilmaz H, Bayindir H. Clinical and scanning electron microscopy evaluation of the Er, Cr: YSGG laser therapy for treating dentine hypersensitivity: short-term, randomised, controlled study. J Oral Rehabil. 2014;41(5):392–398. doi: 10.1111/joor.12156. [DOI] [PubMed] [Google Scholar]
- 26.Pourshahidi S, Ebrahimi H, Mansourian A, Mousavi Y, Kharazifard M. Comparison of Er, Cr: YSGG and diode laser effects on dentin hypersensitivity: a split-mouth randomized clinical trial. Clin Oral Investig. 2019;23(11):4051–4058. doi: 10.1007/s00784-019-02841-z. [DOI] [PubMed] [Google Scholar]
- 27.Gholami GA, Fekrazad R, Esmaiel-Nejad A, Kalhori KA. An evaluation of the occluding effects of Er;Cr:YSGG, Nd:YAG, CO2 and diode lasers on dentinal tubules: a scanning electron microscope in vitro study. Photomed Laser Surg. 2011;29(2):115–121. doi: 10.1089/pho.2009.2628. [DOI] [PubMed] [Google Scholar]
- 28.Loveren C, Schmidlin P, Martens L, Amaechi B. Dentin hypersensitivity management. Clin Oral Investig. 2013;17(Suppl 1):55–59. doi: 10.1007/s00784-012-0912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Douglas-de-Oliveira DW, Vitor GP, Silveira JO, Martins CC, Costa FO, Cota LOM. Effect of dentin hypersensitivity treatment on oral health related quality of life-a systematic review and meta-analysis. J Dent. 2018;71:1–8. doi: 10.1016/j.jdent.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Lima TC, Vieira-Barbosa NM, de Sá Grasielle, Azevedo C, de Matos FR, Douglas de Oliveira DW, de Oliveira ES, et al. Oral health-related quality of life before and after treatment of dentin hypersensitivity with cyanoacrylate and laser. J Periodontol. 2017;88(2):166–72. doi: 10.1902/jop.2016.160216. [DOI] [PubMed] [Google Scholar]
- 31.Parkinson CR, Hughes N, Hall C, Whelton H, Gallob J, Mason S. Three randomized clinical trials to assess the short-term efficacy of anhydrous 0.454% w/w stannous fluoride dentifrices for the relief of dentin hypersensitivity. Am J Dent. 2016;29(1):25–32. [PubMed] [Google Scholar]
- 32.Qin C, Xu J, Zhang Y. Spectroscopic investigation of the function of aqueous 2-hydroxyethylmethacrylate/glutaraldehyde solution as a dentin desensitizer. Eur J Oral Sci. 2006;114(4):354–359. doi: 10.1111/j.1600-0722.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 33.Ozlem K, Esad G, Ayse A, Aslihan U. Efficiency of lasers and a desensitizer agent on dentin hypersensitivity treatment: a clinical study. Niger J Clin Pract. 2018;21(2):225–230. doi: 10.4103/njcp.njcp_411_16. [DOI] [PubMed] [Google Scholar]
- 34.Assis CdAd, Antoniazzi RP, Zanatta FB, Rösing CK. Efficacy of Gluma Desensitizer® on dentin hypersensitivity in periodontally treated patients. Braz Oral Res. 2006;20(3):252–6. doi: 10.1590/S1806-83242006000300013. [DOI] [PubMed] [Google Scholar]
- 35.Sobral M, Garone-Netto N, Luz MAAdC, Santos A. Prevention of postoperative tooth sensitivity: a preliminary clinical trial. J Oral Rehabil. 2005;32(9):661–8. doi: 10.1111/j.1365-2842.2005.01479.x. [DOI] [PubMed] [Google Scholar]
- 36.Mehta D, Gowda VS, Santosh A, Finger WJ, Sasaki K. Randomized controlled clinical trial on the efficacy of dentin desensitizing agents. Acta Odontol Scand. 2014;72(8):936–41. doi: 10.3109/00016357.2014.923112. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed J, Ali SA, Jouhar R, Shah H. Clinical assessment of bonding agent v/s fluoride varnish in dentinal hypersensitivity. JBUMDC. 2019;9(1):53–56. doi: 10.51985/JBUMDC2018022. [DOI] [Google Scholar]
- 38.Aranha ACC, Pimenta LAF, Marchi GM. Clinical evaluation of desensitizing treatments for cervical dentin hypersensitivity. Braz Oral Res. 2009;23(3):333–339. doi: 10.1590/S1806-83242009000300018. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed DEM, Abielhassan MH, Hamza HSE, Haridy MF, Mamdouh M. Sylc air polishing versus fluoride varnish application in managing dentin hypersensitivity in non-carious cervical lesions: a randomized clinical trial. Open Dent J. 2020;14(1):120–126. doi: 10.2174/1874210602014010120. [DOI] [Google Scholar]
- 40.Zhou K, Liu Q, Yu X, Zeng X. Laser therapy versus topical desensitising agents in the management of dentine hypersensitivity: a meta-analysis. Oral Dis. 2021;27(3):422–430. doi: 10.1111/odi.13309. [DOI] [PubMed] [Google Scholar]
- 41.Kumar NG, Mehta D. Short-term assessment of the Nd: YAG laser with and without sodium fluoride varnish in the treatment of dentin hypersensitivity–a clinical and scanning electron microscopy study. J Periodontol. 2005;76(7):1140–1147. doi: 10.1902/jop.2005.76.7.1140. [DOI] [PubMed] [Google Scholar]
- 42.Habdan A, Awdah A, Meshari A, Mokeem L, Saqat R. The effectiveness of Er. Cr. YSGG laser in sustained dentinal tubules occlusion using scanning electron microscopy. J Dent Health Oral Disord Ther. 2017;7(6):00261. doi: 10.15406/jdhodt.2017.07.00261. [DOI] [Google Scholar]
- 43.Aggarwal SD, Borkar A, Borse N, Acharya A. Comparative evaluation of fluoro calcium phosphosilicate, calcium sodium phosphosilicate, and strontium chloride hexahydrate containing dentifrice for the treatment of dentin hypersensitivity: a randomized single-blind study. J Int Oral Health. 2019;11(6):404. doi: 10.4103/jioh.jioh_228_19. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All supporting data are included as additional files, upon request.