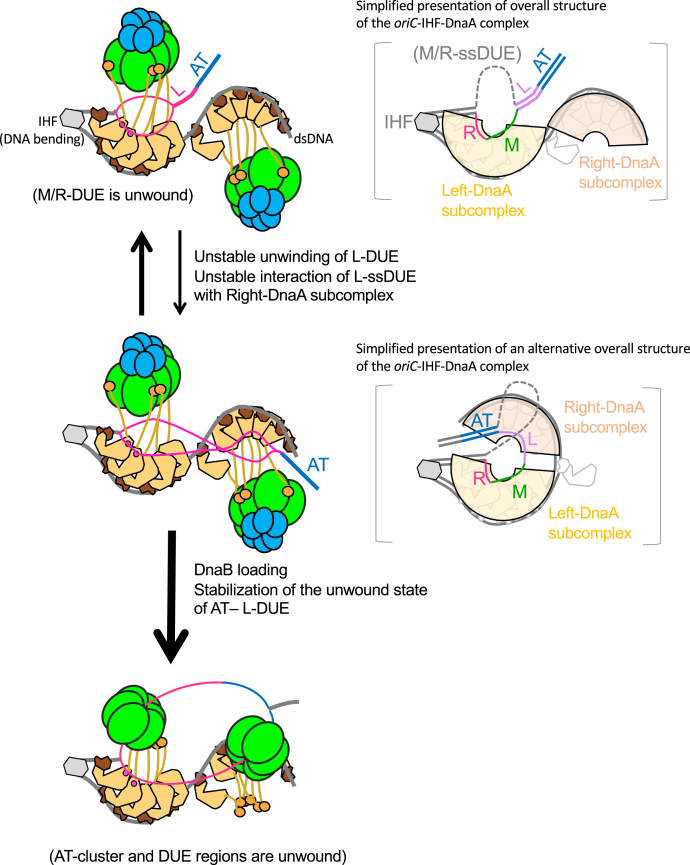
Figure 9.
Model for the concerted action of the DUE and Right-DnaA subcomplex to promote efficient DnaB loading. First, oriC DUE unwinding is promoted by interaction between M/R-DUE and the Left-DnaA subcomplex (Open complex, top left panel). DnaBC complexes are recruited by specific binding of DnaA domain I and DnaB. These complexes are stable and change dynamically to the next complexes by unstable unwinding of L-DUE and unstable interaction of the upper strand of L-DUE and Right-DnaA subcomplex (Expanded open complex, middle left panel). In the right panels with brackets, possible overall structures of the oriC-DnaA-IHF complexes are shown in an alternative simplified manner. DnaBC complexes and DnaA domains I-II are omitted for simplicity. Overall Left/Right-DnaA subcomplexes are surrounded by arches. The two DnaA subcomplexes could swivel using the linker DNA region, bringing the ssDUE and Right-DnaA subcomplex into proximity to facilitate their interaction (right panels). Binding of the Right-DnaA subcomplex to ssDUE could change dynamically, allowing temporal binding to the upper or lower strand of L-DUE region. Unstable unwinding of the AT-cluster region takes over for L-DUE when the Right-DnaA subcomplex is nonfunctional. Two DnaB molecules are loaded onto the lower-strand M/R-DUE region and the upper-strand AT-cluster–L-DUE region via a specific interaction with DnaA domain III (DnaB-loaded complex, bottom panel). DUE, duplex unwinding element; ss, single stranded.