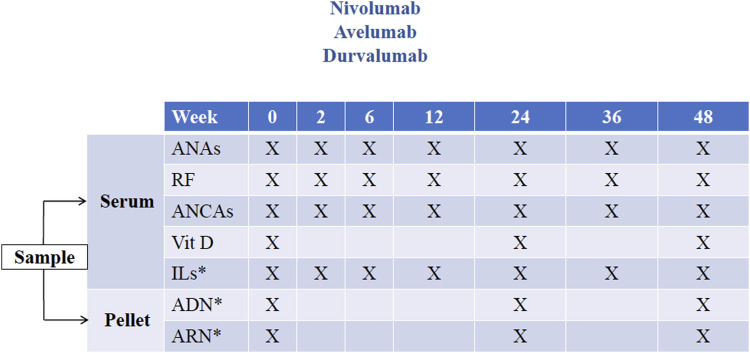
FIGURE 1.
Study schedule 1. Timing of ordinary samples for patients included receiving immune checkpoint inhibitors administered every 2 weeks (nivolumab, avelumab, and durvalumab). *Measurements not included in this study (potentially for use in future research projects). ANA, antinuclear antibody; RF, rheumatoid factor; ANCA, antineutrophil cytoplasmic antibody; Vit D, vitamin D (measured as 25-hydroxycholecalciferol); IL, interleukin.