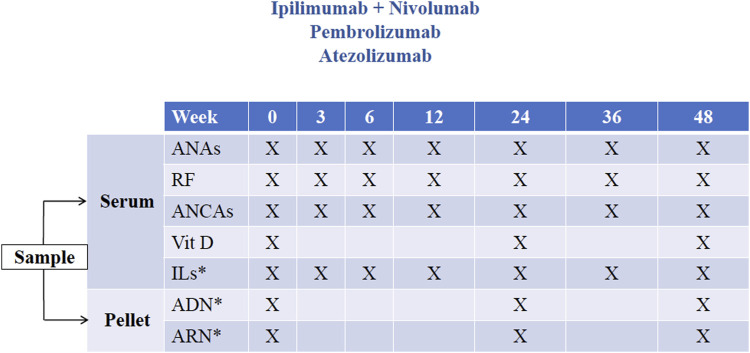
FIGURE 2.
Study schedule 2. Timing of ordinary samples for patients included receiving immune checkpoint inhibitors or combinations of immune checkpoint inhibitors administered every 3 weeks (ipilimumab + nivolumab, pembrolizumab and atezolizumab). *Measurements not included in this study (potentially for use in future research projects). ANA, antinuclear antibody; RF, rheumatoid factor; ANCA, antineutrophil cytoplasmic antibody; Vit D, vitamin D (measured as 25-hydroxycholecalciferol); IL, interleukin.