Abstract
Merkel cell carcinoma (MCC) is a rare and highly aggressive cutaneous neuroendocrine carcinoma. The MCC incidence rate has rapidly grown over the last years, with Italy showing the highest increase among European countries. This malignancy has been the focus of active scientific research over the last years, focusing mainly on pathogenesis, new therapeutic trials and diagnosis. A national expert board developed 28 consensus statements that delineated the evolution of disease management and highlighted the paradigm shift towards the use of immunological strategies, which were then presented to a national MCC specialists panel for review. Sixty-five panelists answered both rounds of the questionnaire. The statements were divided into five areas: a high level of agreement was reached in the area of guidelines and multidisciplinary management, even if in real life the multidisciplinary team was not always represented by all the specialists. In the diagnostic pathway area, imaging played a crucial role in diagnosis and initial staging, planning for surgery or radiation therapy, assessment of treatment response and surveillance of recurrence and metastases. Concerning diagnosis, the usefulness of Merkel cell polyomavirus is recognized, but the agreement and consensus regarding the need for cytokeratin evaluation appears greater. Regarding the areas of clinical management and follow-up, patients with MCC require customized treatment. There was a wide dispersion of results and the suggestion to increase awareness about the adjuvant radiation therapy. The panelists unanimously agreed that the information concerning avelumab provided by the JAVELIN Merkel 200 study is adequate and reliable and that the expanded access program data could have concrete clinical implications. An immunocompromised patient with advanced MCC can be treated with immunotherapy after multidisciplinary risk/benefit assessment, as evidenced by real-world analysis and highlighted in the guidelines. A very high consensus regarding the addition of radiotherapy to treat the ongoing focal progression of immunotherapy was observed. This paper emphasizes the importance of collaboration and communication among the interprofessional team members and encourages managing patients with MCC within dedicated multidisciplinary teams. New insights in the treatment of this challenging cancer needs the contribution of many and different experts.
Keywords: immunotherapy, radiotherapy, skin neoplasms
Background
Merkel cell carcinoma (MCC) is a rare and aggressive cutaneous neuroendocrine carcinoma with disputed origin, high risk of recurrence and aggressive behavior. Only limited therapeutic options for advanced disease are available and clinical management of MCC is quite challenging, continuously evolving across multiple specialties.1
Historically, MCC was first described by Toker2 in 1972 as ‘trabecular carcinoma’ and considered of eccrine origin, while it was identified as neuroendocrine origin on electronic microscopy by Tang and Toker 6 years later.3 Finally, it was newly denominated as MCC from the German histopathologist who discovered special cells of the epidermis in the snout skin of a mole in 1875.4 These putative mechanoreceptors are evident in many species and in the human skin are called Merkel cells. Their similarities to those of the newfound tumor suggested that they are the source of neoplasm.3 However, another hypothesis suggests that MCC originates from a pluripotent dermal stem cell.5 Nowadays, its immunophenotype, very similar to that of the diffused neuroendocrine system, justifies its inclusion among the neuroendocrine neoplasms (NENs) with a skin localization and specific clinical behavior.6 7
Moreover, a special correlation between MCC and the Merkel cell polyomavirus (MCPyV)8 was discovered in 2008. This virus is an enveloped double-stranded DNA polyomavirus that infects most of the human population asymptomatically. However, it has been speculated that in the elderly and/or immunocompromised subjects it can cause MCC. The integrated viral genomes express mutant MCPyV tumor antigens that are crucial for driving the oncogenic development of MCC.9 The virus-related MCC is well distinguished from the non-virus-related MCC, whose pathogenesis is mainly driven by ultraviolet (UV) radiation and whose most frequent mutations are in tumor-suppressor genes such as RB1, TP53 and genes encoding member of Notch family. Therefore, the cancer susceptibility to immune checkpoint inhibition (ICI) led to a novel approach to the treatment of MCC.10 11
Epidemiology
The incidence of MCC is low, ranging from 0.1 to 1.6 cases per 100,000 people per year worldwide.7 However, it has been rapidly increasing over the last 20 years in several countries, especially in Italy, USA, Australia, and Finland. This increase can only be partially explained by improved detection, classification, and coding of MCC.12
The incidence in the Caucasian population exceeds 25 times than that in the other ethnic groups. From a geographical point of view, patients with MCC are fair-skinned, living in areas with high UV B-light indices. The highest incidence, observed in Australia7 is rising at a 4.2% rate annually. Incidence has been growing in Europe as a whole, and in Italy it is especially evident in men.12
The average age at presentation is 69 years old.13 The age-adjusted incidence of MCC in Caucasian patients appears to be linearly correlated to the UV B radiation index.14 The male/female ratio is 2:1 but reports from Finland and China indicate higher incidence in women.15 16 Rare cases were described in pediatric age.17
The most frequently observed localization is in the head and neck, except in patients under 65 years of age and in non-white population. Younger Caucasian patients are more affected in the trunk, while in African Americans the lower extremities are the most common.14 18 In a large analysis of 9387 MCC cases, the most frequent primary site was the head and neck (43%), upper limbs and shoulders (24%), lower limbs and hip (15%), trunk (11%), and other localizations (9%).19
Among the immunosuppressed patients: the risk of developing MCC is approximately 13 times greater in those with HIV infection, 25 times greater in those with organ transplants, and 40 times greater in those with chronic lymphocytic leukemia.20 21 Furthermore, immunosuppressed patients have significantly less survival rate compared with immunocompetent patients.21
MCCs grow and metastasize quickly, about 6%–8% of patients have metastatic disease at the time of diagnosis.22 MCC shows a strong tendency to form satellite lesions in the skin. At the first presentation, while 50%–65% patients show localized disease, 25%–50% have regional metastases and finally about 10% show distant metastases at the time of first presentation.22
Several studies have established the presence of MCPyV in the majority (80%) of MCC23–25 and antibodies that recognize MCPyV oncoproteins are found in approximately 50% of patients with MCC.26
Clinical presentation
Commonly MCC presents itself as a rapidly growing cutaneous or subcutaneous erythematous or violaceous, tender, indurated nodule, or mass sessile or fungating, on sun-exposed areas on the head and neck, frequently ulcerated. A different kind of presentation is represented by papules, plaques, cyst-like structure, pruritic tumors of the lower extremities, pedunculate lesions, subcutaneous masses or telangiectatic papules.27 28
The mucosal form, although rare, has a particularly aggressive behavior and represents a known clinical entity.28
While the skin is generally the primary site of MCC, in very rare cases (up to 4%) of nodal only and/or visceral metastases from MCC without a clear cutaneous MCC primary lesion are described; this is named MCC unknown primary (MCC-UP). This entity seems to be identified as a distinct neuroendocrine carcinoma subtype rather than a variant of cutaneous MCC.22
Metastases could be localized in the skin, lymph nodes, lungs, adrenal glands, pancreas, liver, brain, and bones.29
Prognostic factors
Factors such as the location, the presence or absence of skin lesion, the age of occurrence either under or over 50 years of age, gender, lymph nodes involvement, metastases at diagnosis, the role of the immunodeficiency status and the size either less or greater than 2 cm, which may influence MCC progression are not well defined.30
Overall, survival in MCC depends mainly on the stage at clinical diagnosis31 32: 81% survival rate for stage I; 67% for stage II, 52% for stage III and 11% for stage IV.
Diagnosis
Diagnosis is through clinical examination followed by tissue biopsy33 as the clinical appearance is non-specific and can mimic a variety of benign and malignant skin lesions.
The acronym AEIOU34 summarizes the major features to be considered for the clinical diagnosis: Asymptomatic/lack of tenderness, Expanding rapidly, Immune suppression, Older than 50 years, and Ultraviolet-exposure.
However, the diagnosis is pathological with the finding of small and medium-sized cells expressing neuroendocrine and epithelial markers, synaptophysin, chromogranin-A, cytokeratin 20 and neurofilaments (with dot-like phenotype). Furthermore, at the histological examination, MCC, both positive and negative MCPyV, is distinguished by a rich lymphocytic infiltrate which represents one of the main characteristics of these neoplasms.35 MCC appears as an expansible, nodular, or diffusely infiltrative neoplasm within the dermis, variably in subcutis. It may present a variable mixture of nodules, sheets, nests, and trabeculae of neoplastic cells. An intraepidermal component is occasionally present. The neoplastic MCC cells appear as small, round, blue tumor cells with a high nucleus–cytoplasm ratio, round/oval nuclei, finely dispersed chromatin (salt and pepper), indistinct nucleoli, and scant cytoplasm.29
Ultrasonography and ultrasound-guided fine needle biopsy may be useful for pathological diagnosis and, particularly, for the head and neck localizations.36 37
Staging classification and prognostic stage groups for MCC are based on the American Joint Committee on Cancer (AJCC) staging system, eighth edition. Staging classification includes the location of the primary site (T), a clinical evaluation of regional lymph nodes (clinical N), a pathological evaluation of regional lymph nodes (pathological N), and a definition of clinical and pathological distant metastasis (M). Regarding the prognostic staging, AJCC eighth edition includes six different groups (0, I, IIA, IIB, III, IV) and seven pathological stage groups (0, I, IIA, IIB, IIIA, IIIB, IV) according to the tumor, node, metastasis classification.38
Treatment
Treatment options are based on the stage of the disease. For patients with localized MCC, surgery is the primary treatment modality typically followed by radiotherapy (RT).19 In accordance with the National Comprehensive Cancer Network (NCCN) clinical practice guidelines, the first step for treating early-stage MCC is surgery to remove the primary cancer, followed by sentinel lymph node biopsy and postoperative RT may also be considered.39 Furthermore, it is recommended that patients with stage I and II be treated with radical excision with 1–2 cm margins followed by adjuvant (adj) RT when negative risk factors like lymphovascular invasion or immunosuppression are present. Adj chemotherapy (CT) for stages I–II is not recommended. For patients in stage III or unresectable disease, lymph node dissection and/or RT is generally recommended. The NCCN guidelines also mention that the preferred option for patients with regional disease in stages II and III is the inclusion in clinical trials.39
Correct treatments for early-stage MCC are associated with a good overall survival (OS) and reduced rates of relapse and mortality. A high proportion of patients not adequately and timely treated or immunocompromised, as well as patients with an aggressive MCC progress to metastatic disease. Considering that the prognosis of metastatic MCC (mMCC) is very poor, it is essential that patients with early-stage MCC are treated in the most complete and rapid manner.40 European guidelines in 2015 stated that, apart from surgical removal of isolated metastases, there is no established curative treatment for mMCC.6
Until some years ago the treatment of choice for advanced disease has been CT as well as in poorly differentiated NENs with a median of 3–4 months progression-free survival (PFS), less than median 10 months OS, and significant toxicity.41
Immunotherapy has been extensively studied in MCC in recent years.
To date, different immune checkpoint inhibitors (avelumab, pembrolizumab and nivolumab)42–44 have been shown to be active in the treatment of advanced MCC39: in 2017 the European Commission approved avelumab as monotherapy for the treatment of mMCC, regardless of the line of therapy.45
A Delphi conference has been organized, involving various Italian centers with a focus on metastatic disease, to show how patients with MCC are managed and to share the expertize of all the specialties potentially involved in the clinical management of the disease, such as surgery, immunology, medical oncology, RT, and dermatology, integrating skills and different know-how. The aim is to revise the state of art of MCC, to highlight the Italian expert perspective regarding important issues in clinical practice of management of MCC, and to address some of the limitations of the current NCCN guideline on the topic. Ultimately, this manuscript is a consensus statement on the multidisciplinary management of MCC developed by an Italian group, using a robust methodology, referencing important trials and articles in the field and highlighting areas of ongoing controversy of international interest.
Methods
Consensus statement policy
The Delphi technique, developed in 1962,46 is a process used to achieve a consensus concerning real-world knowledge from experts related to certain areas. The name derives from the Delphic oracle’s skills of interpretation and foresight. Delphi is a well-established methodology used in the scientific field.47–49
The Delphi process traditionally begins with a questionnaire based on an extensive review of the literature; the collected information is converted into another specific questionnaire used as the instrument of the survey. Each Delphi participant is asked to review and rate the summarized statements so that areas of consensus and non-consensus can be identified. In subsequents rounds, each Delphi participant receives a third questionnaire that includes the statements and ratings, and participants are asked to re-evaluate their initial judgment.
Expert board and consensus panel
In December 2020 a multidisciplinary board of 10 experts, based on their documented expertize in the MCC field, met to review the current landscape of the disease and identify key topics for clinical management. The expert board included seven medical oncologists with a wide expertize in NENs, melanomas, and immune oncology, one pathologist, one dermatologist and one plastic surgeon.
At the end of the topic selection process, replies and redundancies were eliminated and 28 items were generated for testing across a wider audience using the Delphi questionnaire. The statements were divided into five areas: multidisciplinary management (4 items), diagnostic pathway (9 items), clinical management aspects (12 items), follow-up (2 items) and guidelines (1 item).
A Likert scale was used (1=no agreement to 7=maximum agreement) to evaluate the degree of agreement with each of the statements proposed in the questionnaire. All activities were coordinated by a facilitator.
All members of the expert board disclosed potential conflicts of interest.
All the Italian specialized centers dedicated to care and management of patients with MCC were contacted and asked for volunteer participation using the following criteria:
Clinical experience: at least 2 years of experience in the field as an attending physician or a consultant.
Clinical research experience: participation in drafting guidelines, or at least one scientific publication or conference abstract in the last 36 months in the field.
Questionnaire
The Delphi questionnaire was submitted via the web. This method granted anonymity, absence of interference among the panelists and safety from the SARS-CoV-2 pandemic. The link to the web was sent by email with a maximum of three reminders. The definitions for consensus and non-consensus were decided a priori.
At the end of the first round (January 15, 2021 to February 4, 2021) the median value and the 25th and 75th percentiles (75th p and 25th p, interquartile range) of each statement were calculated.
In the second round (February 8, 2021 to March 3, 2021), people were asked to answer the same statements considering the interquartile range (IQR) of each question (which represents the range in which 50% of the answers fell) as an index of their colleagues’ responses. Those who answered outside the IQR in the second round were asked to give a reason for their response. At the end of the second round, the median value and the 25th and 75th percentiles of each statement were calculated again.
The results of the first and second rounds and the motivations of those who had answered outside the IQR, were discussed by the expert board at the ‘verification meeting’ (April 2021). After discussing and commenting on the results of each of the 28 statements, the expert board members formulated the counter motivations. Since all 28 statements reached agreement and consensus it was decided not to proceed with the third round. The flow chart of the analysis is presented in figure 1.
Figure 1.
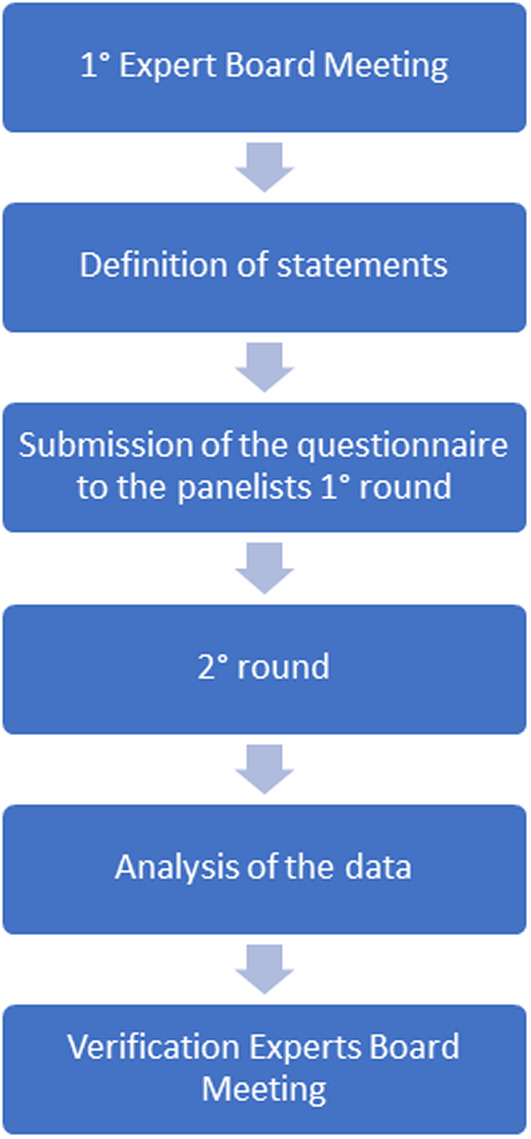
Flow chart of the analysis.
The statements were ranked based on the 25th percentile and the IQR (figure 2):
Figure 2.
Statement ranking.
INDECISION IN THE EVALUATION: Those statements that have the 25th percentile ≥4 and IQR 4–4 or 25th percentile <4 and IQR 3–5 belong to this group.
AGREEMENT AND CONSENT WITH THE STATEMENT: Affirmations that have the 25th percentile ≥4 and IQR ≤2 but different from 4 to 4 belong to this group.
AGREEMENT AND LOW CONSENT WITH THE STATEMENT: Those statements that have the 25th percentile ≥4 and IQR ≥3 belong to this group.
DISAGREE AND CONSENT WITH THE STATEMENT: Those statements that have the 25th percentile <4 and IQR ≤2 belong to this group.
DISAGREE AND LOW CONSENT WITH THE STATEMENT: Those statements that have the 25th percentile <4 and IQR ≥3 belong to this group.
Stata V.16.1 software was used for the analyses.
Results
The statements represent a nationwide expert consensus on the management of patients with MCC. The conclusions of the consensus panel, as set forth in this paper, outlines the panel’s final opinion and should not be used as a substitute for the individual professional judgment of the treating physician.
Participants
Sixty-six panelists answered the questionnaire at the first round, and 65 at the second round.
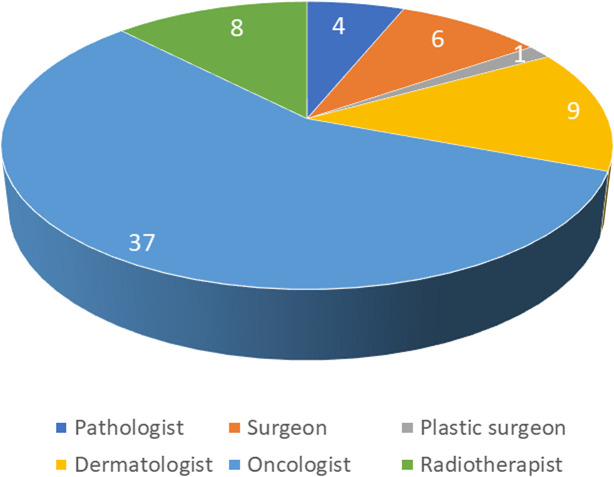
The distribution of the panel was 59.1% oncologists and oncologists/radiotherapists and 40.9% other specializations (pathologist, surgeon, plastic surgeon, dermatologist) (figure 3). With regards to the geographic distribution, the panel was well representative of the Italian situation (51.5% North, 25.8% Center, 22.7% South and Islands). Over 70% of the participants declared over 10 years of experience in MCC field.
Figure 3.
Distribution of the panel by specialization.
Analysis and consensus recommendations
1° area: multidisciplinary management
Box 1. Multidisciplinary management statements.
S1—At my center the most appropriate therapeutic diagnostic path is shared and discussed within a multidisciplinary team.
S2—Faced with new diagnosis of Merkel cell carcinoma (MCC) the therapeutic diagnostic path should be shared by a multidisciplinary team.
S3—The multidisciplinary team should include at least: an oncologist, a surgeon, a dermatologist, a pathologist, a radiotherapist, and a radiologist.
S27—The patient with MCC should be managed within a dedicated network with hub and spoke centers, with reference centers that have a multidisciplinary team.
There was almost total agreement from the first round with the maximum score (7) given by over 70% of the experts in all statements of this area except the first.
For statements 2 and 3, 98% and 97% of panelists, respectively, awarded the maximum score of agreement (7); those who do not give score 7 regarding the need for a path shared by a multidisciplinary team (statement 2), nevertheless assign a high score (6). For statement 27, in the second round we have 100% of experts who give the maximum score of agreement (7) regarding the need for hub and spoke function.
Statement 1 is the one showing relatively more variability. In Round 2, 30% of experts awarded score 6 and 66% experts awarded score 7. One expert awarded score 1 because his center does not have a team.
In this area, subanalyses by geographical area and by specialization do not show any significant variations between geographical areas and a greater consensus among other specialists compared with oncologists.
With regards to S3, the expert board concluded that it should be recommended that a plastic reconstructive surgeon and a nuclear medicine expert be included in the multidisciplinary team. In real life, the multidisciplinary team may not always be represented by all the specialists requested. Therefore, the expert group recommends an initial multidisciplinary discussion to define the right therapeutic strategy as soon as possible.
The panel stated that considering that although MCC is a very rare skin cancer that may initially be noted by the general practitioner or an internist, and referred to a dermatologist for a definitive diagnosis, establishing a multispecialist management immediately after diagnosis is crucial.
2° area: diagnostic pathway
Box 2. Diagnostic pathway statements.
S4—In the minimal criteria for pathological evaluation I believe that the inclusion of the positivity/negativity of polyomavirus is a useful addition.
S5—In the criteria for pathological evaluation I believe that the inclusion of the expression data of cytokeratin 20 (positive) with paranuclear pattern ‘dot-like’ and thyroid transcription factor 1 (negative) is necessary.
S6—In the pathological report, the essential information to be reported is: site, maximum size expressed in cm; extracutaneous extension (bone, muscle, cartilage belt); lymphovascular invasion; state of the deep and peripheral margins; pathological Tumor-Node-Metastasis (pTNM).
S7—In the pathological report it is also strongly recommended to indicate: thickness in mm; mitotic index (number of mitoses/mm2); infiltrated tumor lymphocyte (absent, brisk, not brisk); growth pattern (nodular vs infiltrative).
S8—The following are to be considered as elements for diagnostic framing: age, phototype, center of the lesion, evolution, immunocompromised (transplanted patient, onco-hematologic, positive HIV, with necessity of immunosuppressive therapy).
S9—For proper staging in relation to the site of disease, the imaging tools I consider necessary are CT and/or MRI and/or ultrasound of the lymph node station.
S10—In the preoperative staging I consider the positron emission tomography/CT with 18F-fluorodeoxyglucose necessary.
S11—Sentinel lymph node assessment is necessary for reliable diagnosis/staging in the absence of clinical and/or radiological evidence of lymph node involvement.
S16—The involvement of several lymph node stations belonging to different districts and not radically resectable is considered as a metastatic disease.
Although agreement has been reached since the first round regarding the usefulness of the positivity or negativity of MCPyV in the pathological evaluation (statement 4) there is a certain variability in the responses (therefore a lesser consensus), with 32% of respondents in the second round giving a score of 5. Both the two experts who assign score 4, and the two assigning score 3 provide the justification that the presence of such an indication does not change the therapeutic choice and does not seem to influence the response to treatment. The experts counteract that MCPyV today is not a minimum pathological criterion but could have important strategic therapeutic implications to complete the characterization in the near future. The agreement and the consensus regarding the need for cytokeratin data (statement 5) is greater, with 97% of the experts giving a score of 6 or more at the second round. The experts highlighted that cytokeratin 20 must be positive and the thyroid transcription factor 1 (TTF-1) must be negative to exclude a small cell lung cancer.
Moreover, there is very high agreement and consensus that the site and maximum size of the lesion expressed in ‘cm’ is an essential information for the clinical report (S6), with only one expert in the second round assigning a score of 6 who confirms the usefulness of parameters for prognostic evaluation. Then, about the thickness in ‘mm’ (S7) in the second round, 63% of experts assign 7 and 37% assign 6 with no expert coming outside the IQR range of reference. Statement 8 in fact has all scores equal to 6 or 7, except for a case that assigns 5, but specifying that they are important elements. Statement 9 achieves 100% of votes between 6 and 7. The use of the 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/CT (S10) shows a little more variability (less consensus) in the answers, with 73% of experts assigning a score equal to or greater than 6. One of the reasons for the lower scores is that the 18F-FDG PET/CT does not change the surgical indication if total body CT scan is already negative for distant metastases, and the need for the examination depends on the extent of the primary site and possible lymph node involvement. Although rare, a stage I MCC does not require 18F-FDG PET/CT. The experts counteract that in clinical practice the 18F-FDG PET/CT is proposed when there is a doubt in the radiological imaging interpretation. Among the different experts’ comments, it can be noted that 18F-FDG PET/CT may be overloaded by the need of the double contrast because 18F-FDG PET/CT scan images could underestimate brain, liver and/or lung damage, therefore also requiring CT-related contrast medium. Statement 10 was also analyzed by specialization, highlighting less consensus among oncologists than other specialists.
High consensus and agreement have been reached regarding the need for evaluation of sentinel lymph node (S11), with all votes equal to 6 and 7 except for an expert who assigned 4, deeming it useful but not necessary. The experts highlighted that the evaluation of sentinel lymph node is necessary as it represents an opportunity for in-depth analysis and therapeutic diagnostic algorithm. For the statement number 16, 100% of the panelists awarded a score of 6 or more.
3° area: clinical management
Box 3. Clinical management statements.
12. I always consider the possibility of an adjuvant radiotherapy on the previous site of the primary lesion.
13. I always consider the possibility of an adjuvant lymph node radiation therapy.
14. In case of only lymph node disease, with no evidence of primary cutaneous location and distant metastasis, I would adopt the same therapeutic strategy as the locally advanced Merkel cell carcinoma (MCC) with known primary site.
17. An immunocompromised patient with advanced MCC can be treated with immunotherapy after multidisciplinary risk/benefit assessment in collaboration with the specialists treating him (hematologist, infectiologist, rheumatologist, etc).
18. In a patient with complete clinical-instrumental response I would consider suspending treatment based on the clinical characteristics, response duration and after discussion with the patient.
19. In a patient on immunotherapy I would continue the treatment even beyond progression, in the presence of clinical benefit.
20. In clinical practice I would apply the immune Response Evaluation Criteria in Solid Tumours (RECIST) criteria for the management of patients over time and evaluation under immunotherapy.
21. In the case of curative surgery with demolitive impact, I would discuss with the patient alternative therapeutical medical and/or radiotherapeutic options.
22.The patient with regional recurrence no longer susceptible to radical surgery should be managed with radiotherapy and/or medical therapy (first immunotherapy or if contraindications alternatively chemotherapy).
23. During immunotherapy I would treat the focal progression with the addition of radiotherapy.
24. I consider the design and conduct of the JAVELIN Merkel 200 registration study (part A and part B) with avelumab, entirely adequate to obtain reliable results.
25. I believe that the expanded access program data for avelumab have concrete clinical implications for patient management with metastatic MCC.
The 32% of the experts completely agreed with statement number 12 and a further 28% assigned a score equal to 6, while 50% of the experts expressed a score between 5 and 6 regarding statement 13. In the S12 the analyses based on geographical areas and specializations show a lower agreement between participants from southern Italy and Islands than from the other two geographical areas. A similar observation can be considered between oncologists and the other specializations. A different agreement depending on the geographical area can be noted also for the S13. For both statements, the panelists’ reason for not agreeing fully was that there is no scientific evidence of its usefulness in terms of survival. However, the experts highlighted the need to increase awareness of this topic due to a wide dispersion of the results.
Moreover, 45% are completely in agreement with statement 14 and a further 54% assigned a score of 6, while only one panelist assigned score 5. Sixty-two per cent of the participants are completely in agreement with statement 17 and 37% assigned a score equal to 6, with no differences for geographical area and specialization. Only one panelist assigned score 3, objecting that the data available on safety and efficacy of treating immunocompromised patients with cancer using immunotherapy are limited and many of the clinical trials tend to exclude patients with pre-existing immunodeficiency, thus many of those patients could be undertreated, underdosed, or have an early interruption. The experts replied that several immunocompromised patients were included in the real-world analysis and responded well to the treatment. Avelumab has no flag for immunocompromised patients. This information was also highlighted in the guidelines.
The agreement on treatment discontinuation in a patient with complete instrumental response (S18) is slightly lower than 80% for experts assigning a score equal to or greater than 6, with an interquartile interval ranging from 5 to 6, and 17% of the panelists who assigned 4 placing themselves in a position of ‘impartiality’ with respect to the claim. There is a somewhat lower agreement and consensus in southern Italy than in other geographical areas, while no differences in specialization are noted. The participant scoring 2 affirmed that treatment should be continued, and the expert group agreed that this is an open point. The complete response should be evaluated together with risk/benefit of adverse events and therefore there is a difference between CT and immunotherapy.
About 80% of the panelists assigned a score equal to or greater than 6 to statements 19 and 20. There is a total agreement with regards to statement 21, with 100% scores equal or about 6.
Regarding the statements number 22 and 23 there is an almost total agreement. In the former, 100% of scores are equal to or greater than 6 and in the latter only two experts awarded a score of less than 6 regarding the addition of RT to treat the ongoing focal progression of immunotherapy. The objection was that there is no solid evidence on the usefulness of the addition of RT, but the group of experts replied that it is a rare disease, and more clinical evidence must be gathered.
All (100%) experts agreed that the information concerning avelumab provided by the JAVELIN Merkel 200 study is adequate and reliable and that the expanded access program (EAP) data has concrete clinical implications.
4° area: follow-up
Box 4. Follow-up statements.
15. In the presence of disease without diagnosis of cutaneous primitivity, the prognosis is better overall, at equal stages N and M.
28. I believe clinical radiological follow-up is always needed in patients with Merkel cell carcinoma.
With regards to the best prognosis in the presence of disease without diagnosis of primitivity (S15), 41% of panelists awarded a score of 6 and more and a further 35% assigned a score equal to 5 with an IQR ranging from 5 to 6; 23% assigned 4 placing themselves in a position of ‘impartiality’ with respect to the claim, with no difference between the three groups.
In the second round, 100% of the participants gave the maximum score of agreement 7 regarding the need for clinical radiological follow-up.
5° area: guidelines
Box 5. Guidelines statement.
26. I believe the indications in the national guidelines that refer to the Italian Association of Medical Oncology and international guidelines such as those of the National Comprehensive Cancer Network, are clear and usable.
Only one panelist awarded a less than 6 score for clarity and usability of guidelines. This panelist believes that the NCCN guidelines are more up to date. However, it is important to point out the difference between guideline methodologies: the Italian Association of Medical Oncology (AIOM) guidelines are formulated with specific questions based on the Scottish Intercollegiate Guidelines Network (SIGN) and Grading of Recommendations, Assessment, Development and Evaluations (GRADE) methods.50 51
The NCCN is a non-profit alliance of 31 USA leading cancer centers devoted to patient care, research, education and facilitating quality, effective, efficient, and accessible cancer treatment. NCCN outputs are continuously updated and downloadable. MCC guidelines are available at V.1.2022.39
The AIOM brings together Medical Oncology practitioners to highlight their progress and technical-professional and managerial training; to foster relationships between medical oncologists, and specialists in other disciplines; to establish scientific relations with other Italian and foreign Scientific Societies and Associations; to participate and collaborate with national, regional, and local institutional bodies. In particular, AIOM aims to help develop guidelines (in Italian) for several types of cancer. The latest edition of MCC guidelines was published in 2020 as part of the NENs Guidelines.52
Figure 4 represents the consensus reached at the end of the second round.
Figure 4.
Consensus situation by statement at the end of the second round.
For each statement, the median and IQR are reported for each round, together with the percentage of responses modified in each round. The bar chart and box plot were used to represent the distribution of responses to individual statements for each round (online supplemental file 1).
jitc-2022-004742supp001.pdf (9.9MB, pdf)
Discussion
Several aspects can be derived from this Delphi poll on the clinical management of MCC. First of all, although considered a rare cancer, over the last years a high increase in publications about MCC since 2017 has been observed, probably due to the increase in incidence and the growth in research.
Moreover, the Delphi poll findings demonstrate the importance of collaboration and communication among the interprofessional team to ensure rapid detection, complete work-up, and initiation of appropriate therapy for patients with MCC. Various guidelines recommend in theory a multidisciplinary treatment approach for MCC, including wide local excision, systemic medical therapies, and RT.39
In clinical practice, a multidisciplinary team collaborating in the decision-making process, should be mandatory and established early for the management of patients with MCC. The literature highlights that such approach has a major influence on treatment management in most cases leading to a significant modification of an established treatment plan.53
The panelists agreed that cases of MCC be discussed and referred to dedicated centers as soon as possible. The improvement in new therapeutical opportunities for this rare pathology needs expertize and multidisciplinarily approach. Indeed, the analysis of 5304 cases of MCC at stage I–III of the US national cancer database showed a 62% 5-year survival rate in high-volume centers compared with 56% at lower-volume facilities (p<0.001).54
Considering the limited data available regarding an optimal treatment, a multidisciplinary approach can bring maximum benefit to the management of primary and positive cases of unknown MCPyV.55 Moreover, from a practical point of view, the expertize of a multispecialistic team can be useful in managing the potential immune-mediated events coming from the increased use of immunotherapy.56
With regards to the flow of MCC management there are some objections, among participants due to the absence of solid evidence. It is, however, important to note that the experts knowledge is based on clinical experience. This is why, even if international and national guidelines are considered useful, clear, and usable for all the physicians interviewed as a solid tool to support clinical decisions, they should be integrated by dedicated multidisciplinary clinical expertize.
With reference to diagnostic procedures, from the results of the poll it can be argued that many experts, but not all, are in line with the most up-to-date diagnostic methods such as the immunohistochemistry (IHC) analysis and the nuclear medicine characterization.
IHC is essential to distinguish MCC from its mimics11 19 57; since MCCs are considered neuroendocrine carcinomas which express a set of epithelial and neuroendocrine markers, IHC facilitates the pathological diagnosis. It should be noted that if optical microscopy alone is used, a misdiagnosis risk of around 66% has been reported.30 Characteristically, MCC expresses CK 20, neuroendocrine markers (chromogranin A, synaptophysin, CD56), neurofilament, special AT-rich sequence binding protein-2 (SATB2) and often MCPyV. In most MCCs, tests with positive results for low molecular weight CK (CK8, CK18, CK19 and CK20) can be appreciated, while high molecular weight CK7 is not expressed. Most studies conclude that positive results for CK20 expression along with negative results for CK7 provide an accurate diagnosis in 90% of cases.30 This cytokeratin presents a highly characteristic cytoplasmic paranuclear dot-like pattern and can be considered crucial for the diagnosis. The absence of CK20 expression does not dismiss MCC as diagnosis. Cases of MCC with CK7 +and CK20–cells have also been reported in the literature.30 TTF-1 is a nuclear protein involved in regulating transcriptional activity during embryonic development of the thyroid gland and respiratory epithelium.58 In general, it is positive in small cell lung carcinomas and thyroid cancers and negative in MCC; a negative TTF-1 finding is more specific than a positive CK20 result, because TTF-1 is expressed in very few (3%) MCC, whereas CK20 can be negative in a considerable proportion (20%) of cases.30 Additional markers available to differentiate these neoplasms include SATB2, which has been recently shown to be reactive in MCC.59
In recent years, efforts have been aimed at exploiting the presence of MCPyV oncoproteins in MCCs to develop targeted therapies for virus-positive MCC cancers.60–63 Patients who produce antibodies have a better prognosis compared with antibody-negative patients64; indeed, patients who do not produce antibodies at baseline have an approximately 42% increased risk of recurrence.26 In addition, in patients who have baseline positive MCPyV antibody titers, changes in titers over time reflect changes in MCC disease evolution.26 65 Testing MCPyV antibody can optimize surveillance in conjunction with imaging studies; an increasing titer prompts imaging studies to confirm and localize recurrence in a timely manner, while a decreasing titer indicates that there is no recurrence or that the cancer is reduced significantly.53
Imaging plays a crucial role in diagnosis and initial staging, planning for surgery or radiation therapy, assessment of treatment response and surveillance of recurrence and metastases.66 Moreover, in locally advanced disease or distant metastases, imaging plays an important role in determining the location of the primary tumor and identifying the local–regional invasion to guide surgical or radiation therapy, in particular in the head and neck, due to its anatomic complexity.66
The 18F-FDG is the only PET tracer indicated for imaging MCC, providing high levels of sensitivity and specificity67 for typically highly metabolic neoplasms. Recent studies seem to demonstrate an increased sensitivity of 18F-FDG PET/CT as compared with CT alone for baseline imaging in MCC.37 68 69 MCC may appear hypointense to isointense on T1-weighted sequences and may be isointense to hyperintense on T2/fat saturated T2-weighted images on MRI.70 The hybrid imaging modality 18F-FDG PET/CT—CT is increasingly applied for metastatic or unresectable MCC, providing essential information for staging, re-staging, and treatment monitoring of the disease. Studies in different cancer types have shown that a reduction in FDG uptake is associated with subsequent clinical and radiological responses to immunotherapy.71 The role of 18F-FDG PET/CT in the management of the locoregional MCC is limited, but current evidence suggests a possible significant role even in localized MCC cases.72
Within Delphi participants there was a very high consensus and agreement regarding the need for evaluation of sentinel lymph nodes and the need for clinical radiological follow-up in patients undergoing curative treatments.
Follow-up after locoregional treatment in patients with MCC should encompass a complete physical examination, including skin and imaging examinations for distant metastases (especially for high-risk patients). The complete examination is to be repeated every 3–6 months for the first 3 years.39 Post-definitive treatment surveillance within the first 6 months with 18 F-FDG-PET/CT scan detects unsuspected recurrences early and has prognostic value.73
Given the high frequency of lymph node metastases and the importance of lymph node staging, sentinel lymph node biopsy (SLNB) is highly recommended for patients without clinical and imaging evidence of metastasis. The SLNB positivity has a reported rate ranging from 30% to 38%.39 In SLNB-negative patients, prophylactic RT of the regional nodal basin is not recommended, because it has not been shown to reduce the regional recurrence rate.74 Nevertheless, in patients with a negative SLNB, RT can be indicated if there is an increased recurrence risk (ie, failure to perform appropriate IHC of SLNB, large primary site, chronic immunosuppression).39 Whenever it is possible, basic imaging should be completed prior to the surgical evaluation of lymph nodes in order to define the whole therapeutic strategy in patients with clinically negative node disease, based on the actual extent of the disease.
Surgery is the primary treatment modality for patients with localized MCC, typically followed by RT.19 According to NCCN clinical practice guidelines, current literature on RT advantage is inconsistent, so it is unclear which patients may benefit most from the added therapy.39 In patients with localized disease and negative risk factors, such as lymphovascular invasion or immunosuppression, the NCCN 2021 guidelines39 recommend adj RT of the regional nodal basin. In selected cases in which surgery would result demolithic, causing significant functional or esthetic sequela,75 or is refused by the patient, radiation as monotherapy may be considered. Moreover, the approach could be interesting for elderly patients considering the comorbidities, the decreased muscle mass76 and therefore the risk of anesthesia-related mortality.76 77
In fact, greater dispersion is detectable among participants with regards to the role of adj RT, reflecting the absence of unanimous consent in scientific literature.78 New insights about this topic are expected from the results of STAMP/ECOG 6174 trial (https://clinicaltrials.gov/ct2/show/study/NCT03712605?term=ea6174&rank=1) and ADAM trial (https://clinicaltrials.gov/ct2/show/record/NCT03271372).
Programmed death protein 1 (PD-1) inhibitors such as pembrolizumab and nivolumab and the programmed death-ligand 1 (PD-L1) inhibitor such as avelumab are recommended systemic therapy options for the treatment of the advanced disease based on three phase II international clinical trials, while CT should be discouraged in this setting unless the patient has contraindications to ICI or experienced disease progression on previous treatment with ICI.39 Among the three ICI (avelumab, pembrolizumab, and nivolumab) only avelumab has been approved to date by Food and Drug Administration (FDA) and European Medicines Agency (EMA) for use in the MCC metastatic setting regardless of the line of therapy. Regulatory approval of avelumab for mMCC was based on the JAVELIN Merkel 200 trial (ClinicalTrials.gov: NCT02155647). Part A investigated 88 patients who progressed on prior CT for mMCC: after 1 year follow-up, the objective response rate was 33.0%, with 74% of responses lasting over a year, and the median OS of 12.9 months.79 After 5 years of follow-up, the median OS was 12.6 months, with a 5-year OS rate of 26%.42 Part B of JAVELIN Merkel 200 investigated patients who received no prior systemic therapy for mMCC. The analysis of data included ≥15 months of follow-up for 116 patients, who showed an objective response rate of 39.7%, with the median duration of response 18.2 months; while the median OS was 20.3 months and the 12-month OS rate was 60%, associated with a low rate of grade 3/4 treatment-related adverse events and no treatment-related deaths.80 Since 2018, the NCCN guidelines have recommended avelumab as first line treatment for mMCC.39 The global MCC EAP for avelumab fulfilled an overriding, unmet medical need for patients with mMCC. Before 2017, no treatment was approved for mMCC by the US FDA, European Commission, or any other regulatory authority. Within the large EAP population, avelumab provided a clinical benefit in a real-world population of both immune-competent and immunocompromised patients with mMCC who had various factors that excluded participation in clinical trials. Efficacy and safety data were consistent with pivotal trial data.81 82 Results from Italian patients enrolled in the avelumab EAP were consistent with the findings of the JAVELIN Merkel 200 trial and confirmed the efficacy and safety of avelumab treatment in this population.83 Infusion-related reaction, fatigue, and rashes were among the most frequently occurring and importantly no new safety signals were identified in the EAP population.84
As for the mMCC setting, ICI drastically changed prognosis of a proportion of patients with advanced MCC, as it happened in other oncological fields. Moreover, two phase III (ClinicalTrials.gov Identifier: NCT03712605; NCT03271372), two-arm, trials are currently ongoing in USA to explore how well pembrolizumab and avelumab work compared with standard of care observation in treating patients with stage I–III MCC that has been completely removed by surgery (resected). Immunotherapy with monoclonal antibodies, such as pembrolizumab, may help the body’s immune system attack the cancer, and may interfere with the ability of tumor cells to grow and spread.
For patients who benefit from PD-1/PD-L1 inhibitor, few data exist to define the appropriate duration of ICI therapy after achieving complete response or partial response. Most clinical trials provide treatments for 1–2 years, but a new potential strategy to avoid disease progression after discontinuation of incompletely treated MCC would be to continue ICI administration for a longer period at markedly lower frequency (eg, every 3 months).1 Finally, it should be mentioned that the research on MCC is focusing also on management of patients who progress following anti-PD-1 immunotherapy. This topic has been explored in a phase 2b study with N803, a novel IgG1 Fc-engineered interleukin-15-complexed protein, in addition to an investigator choice ICI in 11 tumor types including MCC. The preliminary data on 135 patients (60% non-small cell lung cancer) show that N803 demonstrates low toxicity in previously treated patients and promising efficacy of cessation of progression, with the potential of induction of response and durable stable disease in patients who had previously progressed.85
The regulatory approval of avelumab addresses an important unmet need in MCC treatment, and in fact 100% of the experts agreed that the information concerning avelumab provided by the JAVELIN Merkel 200 study is adequate and reliable, and that also EAP data have concrete clinical implications.
Patients affected by autoimmune diseases and treated with biologic immunosuppressives, including anti-tumor necrosis factor, are thought to have an increased risk of MCC development86; taking into account a possible cause-effect relationship, after MCC diagnosis immediate discontinuation, at least for a limited time interval, or dose reduction or eventually replacement medication with less immunosuppressive burden should be considered.87
The therapeutic strategy for MCC-UP, that is a deep-seated neoplasm without an associated cancer in the skin and no distant metastases, has not been universally defined to date. Patients with MCC-UP have been observed to have significantly better survival rates compared with MCC of known primary with the same stage of disease.88 89 Efforts have been made to verify if MCC-UP represents a separate disease entity. Recent studies suggest that MCC-UP represents a nodal metastasis from MCCs regressed via enhanced immune function from sun-exposed skin rather than a metastasis from extracutaneous sites or nodal primaries.90 91 In our Delphi poll, in line with the NCCN 2020 guidelines, almost all participants agreed that, in lymph node positivity with no evidence of primary cutaneous location and distant metastases, the same therapeutic strategy of the locally advanced stage with known primary site should be adopted.
Despite recent progress in MCC management across specialties, unmet needs and further research opportunities exist in several areas including improving technology for cancer surveillance, decreasing toxicity of existing therapeutics, reducing incidence of disease recurrence, and advancing novel drugs and therapeutic combination drugs to tackle primary and acquired resistance.
It could be concluded that, since MCC is a rare and heterogeneous cancer, that needs a multidisciplinary approach especially now, with new therapeutic approaches and new drugs, and with the natural history of the disease dramatically changing.
Footnotes
Twitter: @Paolo Antonio Ascierto @PAscierto
Correction notice: This article has been corrected since it was first published. The affiliation of author Vanna Chiarion Sileni has been amended from Veneto Institute of Oncology (IOV) IRCSS, Padova, Italy to Veneto Institute of Oncology (IOV) IRCCS, Padova, Italy.
Collaborators: DELPHI Panel Members: GC. Antonini Cappellini, Oncology Complex Organizational Unit Interhospital Sandro Pertini – Sant’Eugenio – CTO LHU RM2, Rome; L. Antonuzzo, Clinical Oncology Unit, Careggi University Hospital, Florence - Department of Experimental and Clinical Medicine, University of Florence; G. Badalamenti, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo; M. Barberis, Clincal Unit of Histopathology and Molecular Diagnostics IEO, European Institute of Oncology, IRCCS, Milan; F. Bassetto, Plastic Surgery Clinic, Department of Neurosciences, Hospital-University of Padova; R. Berardi, Oncology Clinic Polytechnic University of Marche AOU Ospedali Riuniti of Ancona; A. Berruti, Medical Oncology University of Brescia Department of Medical-Surgical Specialties, Radiological Sciences and Public Health Brescia; A. Bongiovanni, Osteoncology and Rare Tumors Center (CDO-TR), IRCCS Romagnolo Institute for the Study of Tumors (IRST) ‘Dino Amadori’, Meldola, Forlì Cesena; P. Bonomo, Radiation Oncology Hospital University Azienda Careggi, Florence; L. Borgognoni, Division of Plastic and Reconstructive Surgery, Melanoma & Skin Cancer Unit, SM Annunziata Hospital, Florence; V. Borzillo, Radiation Oncology Unit Istituto Nazionale Tumori IRCCS Fondazione ‘G. Pascale’, Napoli; F. Bruder, Acting Head of Medical Oncology Complex Structure Businco (AOB), Cagliari; D. Campana, Division of Medical Oncology, IRCCS Hospital University Azienda of Bologna; F. Consoli, Organizational Unit Medical Oncology, ASST Spedali Civili, Brescia; A. Cordova, Department of Oncological and Stomatological Surgical Disciplines University of Palermo; R. Depenni Department of Oncology, AOU Policlinico of Modena; A.M. Di Giacomo, University of Siena, Center for Immuno-Oncology, University Hospital of Siena; G. Fabbrocini, Director of the Complex Organizational Unit of Clinical Dermatology of the AOU Federico II of Naples; M.C. Fargnoli, Dermatology, Department of Biotechnological and Applied Clinical Sciences, University of L’Aquila; V. Ferraresi, Sarcomas and Rare Tumors Departmental Unit IRCCS Regina Elena National Cancer Institute of Rome; F. Ferrau, Director, Oncology Complex Organizational Unit, ‘S.Vincenzo’ Hospital, Taormina, Messina; M.T. Fierro, Dermatologic Clinic, Dept Medical Sciences, University of Turin; M. Gatti, Acting Director of Radiotherapy Candiolo Cancer Institute, FPO - IRCCS, Candiolo Turin; F. Gelsomino, Department of Oncology and Hematology, Division of Oncology, University Hospital of Modena; D. Giuffrida, Oncology, Mediterranean Oncological Institute, Viagrande, Catania; P. Graziano, Director Unit of Pathology Fondazione IRCCS Osp.Casa Sollievo della Sofferenza, S.Giovanni Rotondo, Foggia; C. Gregorelli, Plastic and Reconstructive Surgery of the Civil Hospitals of Brescia; M. Guida, Rare Tumors and Melanoma Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Tumori Giovanni Paolo II, Bari; D. Massi, Section of Pathology, Department of Health Sciences, University of Florence; R. Mazzarotto, Department of Radiation Oncology, Verona University Hospital, Verona; L. Milesi, Complex Organizational Unit Oncology ASST Papa Giovanni XXIII Bergamo; AM. Minisini, University Health Unit Friuli Centrale Oncology Department Udine; F. Morgese, Oncology Clinic AOU Ospedali Riuniti Ancona; P. Muto, Department of Radiation Oncology, Istituto Nazionale Tumori - IRCCS - Fondazione G. Pascale, Napoli; G. Palmieri, Immuno-Oncology & Targeted Cancer Biotherapies, University of Sassari - Unit of Cancer Genetics, IRGB-CNR, Sassari; R. Patuzzo, Melanoma and Sarcoma Complex Structure Unit-IRCCS Foundation National Cancer Institute Milan; G. Pellacani, Dermatology Clinic, Department of Clinical Internal, Anesthesiological and Cardiovascular Sciences, University of Rome La Sapienza; F. Picciotto, Dermatologic Surgery Section Dept. of Surgery A.O.U. Città della Salute e della Scienza, Torino; J. Pigozzo, Melanoma Oncology Unit Veneto Institute of Oncology IOV – IRCCS, Padua; N. Pimpinelli, Dermatology Unit, Dept. Health Sciences, University of Florence; P. Poletti, Oncology Complex Organizational Unit Papa Giovanni XXIII Hospital, Bergamo; L. Repetto, Director of the Oncology Department Director Sc. Oncology G. Borea Hospital Sanremo (Imperia) LHU 1 Health System Liguria Region; G. Rinaldi Medical Oncology Complex Organizational Unit UOP P. Giaccone Palermo; P. Rubegni, Dermatology Unit Department of Medical, Surgical and Neuroscience University of Siena; G. Rubino, Unit of Radiation Oncology, Oncology Department, University Hospital of Siena; F. Spagnolo, Medical Oncology 2, IRCCS San Martino Polyclinic Hospital, Genoa; L. Tagliaferri, Complex Organizational Unit of Oncological Radiotherapy, Department of Diagnostic Imaging, Oncological Radiotherapy and Hematology, ‘A. Gemelli’ University Hospital Foundation IRCCS, Rome; E. Tanda, Medical Oncology, Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS) Ospedale Policlinico San Martino, Genova, Genetics of Rare Cancers, Department of Internal Medicine and Medical Specialties, University of Genoa; M.C. Tronconi, Medical Oncology and Hematology Unit, Humanitas Cancer Center, Humanitas Clinical and Research Center, IRCCS, Rozzano, Milan; M. Tucci, Interdisciplinary Department of Medicine University of Bari ‘Aldo Moro’ and IRCCS - National Tumori Institute Giovanni Paolo II, Bari; M. Valente, Center for Immuno-Oncology B Medical Oncology and Immunotherapy Division Oncology Department University Hospital of Siena; B. Vincenzi, Associate Professor in Medical Oncology University Campus Bio-Medico, Rome; I. Zalaudek, Department of Dermatology and Venereology, University of Trieste.
Contributors: The authors took full responsibility for the content of this publication and confirmed that it reflects their viewpoint and expertise. All authors provided reviews and edits, ensured accuracy of the consensus opinions, and have read and approved the final version of this manuscript.
Funding: The development of this publication was financially supported by Merck Serono S.p.A., Rome, Italy, an affiliate of Merck KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945) and Pfizer through an independent medical writing grant. The views and opinions described in this publication do not necessarily reflect those of the grantor. Medical writing services were provided by Fullcro Srl.
Competing interests: PAA—received research funding from Bristol Myers Squibb, Roche-Genentech, Pfizer/Array, Sanofi; had a consultant role for Bristol Myers Squibb, Roche-Genentech, Merck Sharp & Dohme, Novartis, Merck Serono, Pierre-Fabre, Sun Pharma, Sanofi, Idera, Sandoz, 4SC, Italfarmaco, Nektar, Pfizer/Array, Lunaphore, Medicenna; participated to Advisory Board for Bristol Myers Squibb, Roche-Genentech, Merck Sharp & Dohme, Novartis, AstraZeneca, Immunocore, Boehringer-Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Oncosec, Nouscom, Seagen, iTeos. PB—Advisory board or conference honoraria: Merck, Sanofi-Regeneron, Merck Sharp & Dohme, Sun Pharma, Angelini, Molteni, Bristol Myers Squibb, GSK, Nestlè. CC—Participation to scientific meetings provided by Novartis, Sun, Pierre-Fabre, Bristol Meyers Squibb; Advisory board participation Novartis, MSD. VCS—Participation to scientific meetings provided by Novartis, Sun, Pierre-Fabre, Bristol Meyers Squibb; Advisory board participation Merck-Serono, Pierre-Fabre, Novartis; Invited speaker: Pierre-Fabre, Novartis, Bristol Meyers Squibb. APDT—Advisory Boards Bayer, Roche, Novartis, PharmaMar. NF—Personal financial interest Advisory board: AAA, Merck, MSD, Novartis, Pfizer; Public speaking: AAA, Ipsen. Institutional financial interest Grant: Ipsen, AAA, Merck. Clinical trials (local PI): 4SC, Astellas, Beigene, Fibrogen, Incyte, Ipsen, MSD, Nucana. GG—Participation to board: Merck, Glaxo, Pharmamar, Novartis, Bayer, EISAI. MM—Honoraria for serving as a speaker from MSD, Roche, Bristol Myers Squibb (BMS), Sanofi, AstraZeneca, Amgen, Pierre Fabre, Eli Lilly, GlaxoSmithKline (GSK), Sciclone, Alfasigma, and Merck Serono; Personal fees for advisory boards from Merck Sharp & Dohme Corp., Roche, BMS, Incyte, AstraZeneca, Amgen, Pierre Fabre, Eli Lilly, Sanofi, GSK, Alfasigma, and Merck Serono; Stockholder in Epigen Therapeutics and Theravance. PQua—Invited speaker and advisory board participation: BMS, Merck, MSD; Novartis, Pierre Fabre, Sanofi, Roche. PQue—Advisory board participation: Roche, Novartis, Pierre Fabre, Merk Sharp and Dome, BMS, Sanofi, Sun Pharma, Merck. FS—Personal financial interests Advisory board, public speaking: Ipsen, Novartis, Pfizer, Advanced Accelerator Applications, Merck, Hutchison Medipharma Limited—Institutional financial interests Clinical trials (PI): GETNE, Incyte, MSD, Hutchison Medipharma Limited—Non financial interests AIOM: coordinator of neuroendocrine neoplasms guidelines, ITANET: executive board member ESMO: member of the NETs and endocrine tumors faculty Panelists MB reports honoraria for consulting, advisory role, speakers’ bureau, travel, accommodation, expenses from MSD, Roche/Genertech, AstraZeneca, Thermo Fisher Scientific, Illumina. FB reports participation to Advisory Klox Technologies, Smith And Nephew, Acelity 3m, Urgo Medical, Decomed, Mimedx. RB reports donors to the Hospital and/or participation to Advisory Boards AZ, BI, Novartis, MSD, Otsuka, Lilly, Roche, Amgen, GSK, Eisai. AB reports advisory board: Janssen, IPSEN, Novartis AAA, AMGEN - Public speaking: Novartis AAA, AMGEN. DC reports consultant/advisory role for Ipsen, Novartis, Pfizer, Advanced Accelerator Applications, Merck. FC reports advisory board or conference honoraria for BMS, MSD, Pierre Fabre, Novartis. RD reports participation as advisor and speaker for BMS, Novartis, MSD, Sanofi, Pierre Fabre. AMDG has served as a consultant and/or advisor to Incyte, Pierre Fabre, Glaxo Smith Kline, Bristol Myers Squibb, Merck Sharp Dohme, and Sanofi and has received compensated educational activities from Bristol Myers Squibb, Merck Sharp Dohme, Pierre Fabre and Sanofi. GF reports AbbVie Srl, Ag Global Event, Allmiral, Amgen, Atenapoli, Bb & C. Srl, Boehringer Ingelheim Italia Spa, Casa Di Cura Ruesh Spa, Collage Spa, Cosmetica Srl, Defhaforum Srl, Dialecticon Srl, Dynamico Education Srl, Editamed Srl, Edizioni Minerva Medica, Effetti Srl, Eli Lilly Italy Spa, F.C. Congressi Srl, Galderma Italia Spa, General Topica Srl, Havas Life Italy Srl, Havas Pr Milan Srl, Health Publishing, Healthware Group, Hippocrates Sintech Srl, Hps Health Publishing & Service Srl, Icim International Srl, Infomedica Srl, Intramed Comunication, Iqvia Solution Srl, Janssen Cilag Srl, La Fonderie, Ldv Srl, Leo Pharma Spa, Maghia Srl, Medica Editoria, Medicoop Vesevo, Mertz Pharma Italia Spa, Micom Srl, Non Solo Meeting Srl, Novartis Pharma Ag, Palazzo Della Salute Srl, Perrigo Italia Srl, Pierre Fabre Dermo Cosmetique, Plannig Congressi, Prex Srl, Qbggroup Srl, Sidemast Srl, Sifarma Spa con socio unico, Sifarma Spa, Sintesi Infomedica Srl, Tecniche Nuove Spa, Thenewway Srl, Triumph Italy Srl, Uvet Gbt Spa, Valetudo Srl, Vyvalife Srl, Vyvamed Srl, Winch ‐ Srl. MCF has served on advisory boards and received honoraria for lectures from Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Sanofi and Sun Pharma. MTF reports participation to scientific meetings provided by Novartis, Pierre-Fabre, Bristol Meyers, Lilly, AbbVie. FG received honoraria for speaker/advisory role from Servier, Eli Lilly, Iqvia, Merck Serono, Amgen and Bristol Myers Squibb. DG reports Pfizer, Ipsen, Merck. PG reports lecture fees and Advisory Board: AstraZeneca, Roche, Pfizer, MSD, Novartis, Eli Lilly, Boehringer Ingelheim, AMGEN. MG reports participation to scientific meetings provided by Merck Sharp & Dohme, Novartis, Sun, Pierre-Fabre, Bristol Meyers Squibb; advisory board participation for Pierre-Fabre, Novartis, Merck Sharp & Dohme, Bristol Meyers Squibb; invited speaker: Pierre-Fabre, Novartis, Merck Sharp & Dohme, Bristol Meyers Squibb. DM reports honoraria or Advisory Board for Novartis, Bayer HealthCare Pharmaceuticals, Pierre-Fabre, Sanofi Genzyme, MSD Italia S.r.l., Roche, Skyline Dx B.V. LM reports participation to board Astellas and Ipsen. AMM reports Novartis, BMS, MSD, Pierre Fabre, Gilead, Sunpharma, Merck’s, Sanofi. FM reports consultant in events organized by Bristol Meyers Squibb, MSD, Sanofi, Sunpharma, Novartis e Pierre-Fabre, honoraria for participation to events MSD, Sanofi, Sunpharma, Novartis e Pierre-Fabre, participation to meetings: Pharmar, BMS, Novartis - Advisory Board Bristol Meyers Squibb, MSD, Sanofi, Sunpharma, Novartis e Pierre-Fabre. GP reports invited speaker and advisory board participation: BMS, MSD, Incyte, Novartis, Pierre Fabre, Roche, Sanofi. GP reports AbbVie; Almirall; Janssen Cilag; Leo-Pharma; Merck Serono, Novartis, Pfizer, Pierre Fabre, Sanofi Genzyme. JP reports participation to advisory board BMS, MSD. NP reports Kyowa Kirin, Merck Serono, MSD, Novartis, Pierre Fabre, Recordati Rare Diseases, Sanofi, Takeda. FS reports lecture fees: BMS, Novartis, MSD, Pierre Fabre, Sun Pharma, Sanofi, Merck, advisory board: MSD, Novartis, Philogen, Sun Pharma. LT reports financial support with the following subjects with commercial interests in the health sector: Elekta, Igea Medical, Sun Farma, Sanofi, Roche, Molipharma, Nanobiotix AB, HNS GEC-ESTRO WG, AIRO. IZ reports Advisory Board or speakers fee from Novartis, Sanofi Genzyme, Sunpharma, Philogen, MSD, La Roche Posay, Biogena. GCAC, LA, GB, AB, PB, LB, VB, FB, AC, VF, FF, MG, CG, RM, PM, RP, FP, PP, LR, GR, PR, GR, ET, MCT, MT, MV, BV have declared no significant conflict of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
DELPHI Panel Members:
GC Antonini Cappellini, L Antonuzzo, G Badalamenti, M Barberis, F Bassetto, R Berardi, A Berruti, A Bongiovanni, P Bonomo, L Borgognoni, V Borzillo, F Bruder, D Campana, F Consoli, A Cordova, R Depenni, AM Di Giacomo, G Fabbrocini, MC Fargnoli, V Ferraresi, F Ferrau, MT Fierro, M Gatti, F Gelsomino, D Giuffrida, P Graziano, C Gregorelli, M Guida, D Massi, R Mazzarotto, L Milesi, AM Minisini, F Morgese, P Muto, G Palmieri, R Patuzzo, G Pellacani, F Picciotto, J Pigozzo, N Pimpinelli, P Poletti, L Repetto, G Rinaldi, P Rubegni, G Rubino, F Spagnolo, L Tagliaferri, E Tanda, MC Tronconi, M Tucci, M Valente, B Vincenzi, and I Zalaudek
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Park SY, Doolittle-Amieva C, Moshiri Y, et al. How we treat Merkel cell carcinoma: within and beyond current guidelines. Future Oncol 2021;17:1363. 10.2217/fon-2020-1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Toker C. Trabecular carcinoma of the skin. Arch Dermatol 1972;105:107. [PubMed] [Google Scholar]
- 3. Tang CK, Toker C. Trabecular carcinoma of the skin: an ultrastructural study. Cancer 1978;42:2311–21. [DOI] [PubMed] [Google Scholar]
- 4. Merkel F. Tastzellen und Tastkörperchen bei den Hausthieren und beim Menschen. Archiv F Mikrosk Anat 1875;11:636–52. 10.1007/BF02933819 [DOI] [Google Scholar]
- 5. Sibley RK, Dehner LP, Rosai J. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. I. A clinicopathologic and ultrastructural study of 43 cases. Am J Surg Pathol 1985;9:95–108. 10.1097/00000478-198502000-00004 [DOI] [PubMed] [Google Scholar]
- 6. Lebbe C, Becker JC, Grob J-J, et al. Diagnosis and treatment of Merkel cell carcinoma. European consensus-based interdisciplinary guideline. Eur J Cancer 2015;51:2396–403. 10.1016/j.ejca.2015.06.131 [DOI] [PubMed] [Google Scholar]
- 7. Schadendorf D, Lebbé C, Zur Hausen A, et al. Merkel cell carcinoma: epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer 2017;71:53–69. 10.1016/j.ejca.2016.10.022 [DOI] [PubMed] [Google Scholar]
- 8. Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008;319:1096–100. 10.1126/science.1152586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu W, You J. Merkel cell polyomavirus and human Merkel cell carcinoma. Recent Results Cancer Res 2021;217:303–23. 10.1007/978-3-030-57362-1_12 [DOI] [PubMed] [Google Scholar]
- 10. Uchi H. Merkel cell carcinoma: an update and immunotherapy. Front Oncol 2018;8:48. 10.3389/fonc.2018.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Becker JC, Stang A, DeCaprio JA, et al. Merkel cell carcinoma. Nat Rev Dis Primers 2017;3:17077. 10.1038/nrdp.2017.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stang A, Becker JC, Nghiem P, et al. The association between geographic location and incidence of Merkel cell carcinoma in comparison to melanoma: an international assessment. Eur J Cancer 2018;94:47–60. 10.1016/j.ejca.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mazzoni E, Rotondo JC, Marracino L, et al. Detection of Merkel cell polyomavirus DNA in serum samples of healthy blood donors. Front Oncol 2017;7:294. 10.3389/fonc.2017.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agelli M, Clegg LX, Becker JC, et al. The etiology and epidemiology of Merkel cell carcinoma. Curr Probl Cancer 2010;34:14–37. 10.1016/j.currproblcancer.2010.01.001 [DOI] [PubMed] [Google Scholar]
- 15. Kukko H, Böhling T, Koljonen V, et al. Merkel cell carcinoma - a population-based epidemiological study in Finland with a clinical series of 181 cases. Eur J Cancer 2012;48:737–42. 10.1016/j.ejca.2011.06.001 [DOI] [PubMed] [Google Scholar]
- 16. Howlader N, Shiels MS, Mariotto AB, et al. Contributions of HIV to non-hodgkin lymphoma mortality trends in the United States. Cancer Epidemiol Biomarkers Prev 2016;25:1289–96. 10.1158/1055-9965.EPI-16-0273 [DOI] [PubMed] [Google Scholar]
- 17. Marzban S, Geramizadeh B, Farzaneh M-R. Merkel cell carcinoma in a 17-year-old boy, report of a highly aggressive fatal case and review of the literature. Rare Tumors 2011;3:108–10. 10.4081/rt.2011.e34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fitzgerald TL, Dennis S, Kachare SD, et al. Dramatic increase in the incidence and mortality from Merkel cell carcinoma in the United States. Am Surg 2015;81:802–6. 10.1177/000313481508100819 [DOI] [PubMed] [Google Scholar]
- 19. Harms PW, Harms KL, Moore PS, et al. The biology and treatment of Merkel cell carcinoma: current understanding and research priorities. Nat Rev Clin Oncol 2018;15:763–76. 10.1038/s41571-018-0103-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garrett GL, Blanc PD, Boscardin J, et al. Incidence of and risk factors for skin cancer in organ transplant recipients in the United States. JAMA Dermatol 2017;153:296–303. 10.1001/jamadermatol.2016.4920 [DOI] [PubMed] [Google Scholar]
- 21. Cook L. Polyomaviruses. Microbiol Spectr 2016;4:197–216. 10.1128/microbiolspec.DMIH2-0010-2015 [DOI] [PubMed] [Google Scholar]
- 22. Harms KL, Healy MA, Nghiem P, et al. Analysis of prognostic factors from 9387 Merkel cell carcinoma cases forms the basis for the new 8th edition AJCC staging system. Ann Surg Oncol 2016;23:3564–71. 10.1245/s10434-016-5266-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu W, Krump NA, Buck CB. Merkel cell polyomavirus infection and detection. J Vis Exp 2019;144:10.3791/58950. 10.3791/58950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barreira JV, Valejo Coelho MM, Ribeiro C, et al. Unknown primary Merkel cell carcinoma with cutaneous spread. BMJ Case Rep 2019;12:224834. 10.1136/bcr-2018-224834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baez CF, Gonçalves MTV, da Rocha WM, et al. Investigation of three oncogenic epitheliotropic viruses shows human papillomavirus in association with non-melanoma skin cancer. Eur J Clin Microbiol Infect Dis 2019;38:1129–33. 10.1007/s10096-019-03508-z [DOI] [PubMed] [Google Scholar]
- 26. Paulson KG, Lewis CW, Redman MW, et al. Viral oncoprotein antibodies as a marker for recurrence of Merkel cell carcinoma: a prospective validation study. Cancer 2017;123:1464–74. 10.1002/cncr.30475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blumenthal L, VandenBoom T, Melian E, et al. Multiple primary Merkel cell carcinomas presenting as pruritic, painful lower leg tumors. Case Rep Dermatol 2015;7:316–21. 10.1159/000441412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nambudiri VE, Vivero M, Watson AJ, et al. Merkel cell carcinoma presenting as subcutaneous breast masses: an uncommon presentation of a rare neuroendocrine neoplasm. Breast J 2016;22:113–5. 10.1111/tbj.12534 [DOI] [PubMed] [Google Scholar]
- 29. Dellambra E, Carbone ML, Ricci F. Merkel cell carcinoma. Biomedicines 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Llombart B, Kindem S, Chust M. Merkel cell carcinoma: an update of key imaging techniques, prognostic factors, treatment, and follow-up. Actas Dermosifiliogr 2017;108:98–107. 10.1016/j.ad.2016.07.021 [DOI] [PubMed] [Google Scholar]
- 31. Allen PJ, Busam K, Hill AD, et al. Immunohistochemical analysis of sentinel lymph nodes from patients with Merkel cell carcinoma. Cancer 2001;92:1650–5. [DOI] [PubMed] [Google Scholar]
- 32. Tai P. Merkel cell cancer: update on biology and treatment. Curr Opin Oncol 2008;20:196–200. 10.1097/CCO.0b013e3282f46d5d [DOI] [PubMed] [Google Scholar]
- 33. Patel P, Hussain K. Merkel cell carcinoma. Clin Exp Dermatol 2021;46:814–9. 10.1111/ced.14530 [DOI] [PubMed] [Google Scholar]
- 34. Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol 2008;58:375–81. 10.1016/j.jaad.2007.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fried I, Cerroni L. [Merkel cell carcinoma]. Pathologe 2014;35:467–75. 10.1007/s00292-014-1935-x [DOI] [PubMed] [Google Scholar]
- 36. Peloschek P, Novotny C, Mueller-Mang C, et al. Diagnostic imaging in Merkel cell carcinoma: lessons to learn from 16 cases with correlation of sonography, CT, MRI and PET. Eur J Radiol 2010;73:317–23. 10.1016/j.ejrad.2008.10.032 [DOI] [PubMed] [Google Scholar]
- 37. Hawryluk EB, O'Regan KN, Sheehy N, et al. Positron emission tomography/computed tomography imaging in Merkel cell carcinoma: a study of 270 scans in 97 patients at the Dana-Farber/Brigham and women's cancer center. J Am Acad Dermatol 2013;68:592–9. 10.1016/j.jaad.2012.08.042 [DOI] [PubMed] [Google Scholar]
- 38. Cates JMM. The AJCC 8th edition staging system for soft tissue sarcoma of the extremities or trunk: a cohort study of the seer database. J Natl Compr Canc Netw 2018;16:144–52. 10.6004/jnccn.2017.7042 [DOI] [PubMed] [Google Scholar]
- 39. Donigan JM, Farma JM, Fung MA. NCCN guidelines version 1.2022 Merkel cell carcinoma, 2021. Available: https://www.nccn.org/home/ [Accessed 23 Nov 2021].
- 40. Palencia R, Sandhu A, Webb S, et al. Systematic literature review of current treatments for stage I-III Merkel cell carcinoma. Future Oncol 2021;17:4813–22. 10.2217/fon-2021-0574 [DOI] [PubMed] [Google Scholar]
- 41. Garcia-Carbonero R, Marquez-Rodas I, de la Cruz-Merino L, et al. Recent therapeutic advances and change in treatment paradigm of patients with Merkel cell carcinoma. Oncologist 2019;24:1375–83. 10.1634/theoncologist.2018-0718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. D'Angelo SP, Bhatia S, Brohl AS, et al. Avelumab in patients with previously treated metastatic Merkel cell carcinoma (JAVELIN Merkel 200): updated overall survival data after >5 years of follow-up. ESMO Open 2021;6:100290. 10.1016/j.esmoop.2021.100290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nghiem P, Bhatia S, Lipson EJ, et al. Three-year survival, correlates and salvage therapies in patients receiving first-line pembrolizumab for advanced Merkel cell carcinoma. J Immunother Cancer 2021;9. 10.1136/jitc-2021-002478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Topalian SL, Bhatia S, Hollebecque A. Abstract CT074: Non-comparative, open-label, multiple cohort, phase 1/2 study to evaluate nivolumab (NIVO) in patients with virus-associated tumors (CheckMate 358): efficacy and safety in Merkel cell carcinoma (MCC). Am Assoc Cancer Res 2017:CT074. [Google Scholar]
- 45. Merck KGaA Darmstadt G. Avelumab Summary of product characteristics. 2021.
- 46. Dalkey N, Helmer-Hirschberg O. An experimental application of the Delphi method to the use of experts. Santa Monica, CA, 1962. Available: https://www.rand.org/content/dam/rand/pubs/research_memoranda/2009/RM727.1.pdf [Accessed 28 Apr 2017].
- 47. Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs 2000;32:1008–15. [PubMed] [Google Scholar]
- 48. Nair R, Aggarwal R, Khanna D. Methods of formal consensus in classification/diagnostic criteria and Guideline development. Semin Arthritis Rheum 2011;41:95–105. 10.1016/j.semarthrit.2010.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol 2014;67:401–9. 10.1016/j.jclinepi.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 50. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brunetti M, Shemilt I, Pregno S, et al. GRADE guidelines: 10. Considering resource use and rating the quality of economic evidence. J Clin Epidemiol 2013;66:140–50. 10.1016/j.jclinepi.2012.04.012 [DOI] [PubMed] [Google Scholar]
- 52. AIOM in condivisione con It.a.net, Coordinatrice: Francesca Spada. Linee guida NEOPLASIE NEUROENDOCRINE, 2020. Available: https://www.aiom.it/wp-content/uploads/2020/10/2020_LG_AIOM_Neuroendocrini.pdf [Accessed 29 Nov 2021].
- 53. Song Y, Azari FS, Tang R, et al. Patterns of metastasis in Merkel cell carcinoma. Ann Surg Oncol 2021;28:519–29. 10.1245/s10434-020-08587-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yoshida EJ, Luu M, Freeman M, et al. The association between facility volume and overall survival in patients with Merkel cell carcinoma. J Surg Oncol 2020;122:254–62. 10.1002/jso.25931 [DOI] [PubMed] [Google Scholar]
- 55. Zerini D, Patti F, Spada F, et al. Multidisciplinary team approach for Merkel cell carcinoma: the European Institute of oncology experience with focus on radiotherapy. Tumori 2021;107:145–9. 10.1177/0300891620944209 [DOI] [PubMed] [Google Scholar]
- 56. Monteiro A, Gouveia E, Garcez D, et al. Challenges of new approaches in metastatic Merkel cell carcinoma. Case Rep Oncol 2020;13:501–7. 10.1159/000507279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Coggshall K, Tello TL, North JP, et al. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol 2018;78:433–42. 10.1016/j.jaad.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 58. Bickle K, Glass LF, Messina JL, et al. Merkel cell carcinoma: a clinical, histopathologic, and immunohistochemical review. Semin Cutan Med Surg 2004;23:46–53. 10.1016/s1085-5629(03)00087-7 [DOI] [PubMed] [Google Scholar]
- 59. Bellizzi AM. SATB2 in neuroendocrine neoplasms: strong expression is restricted to well-differentiated tumours of lower gastrointestinal tract origin and is most frequent in Merkel cell carcinoma among poorly differentiated carcinomas. Histopathology 2020;76:251–64. 10.1111/his.13943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chapuis AG, Afanasiev OK, Iyer JG, et al. Regression of metastatic Merkel cell carcinoma following transfer of polyomavirus-specific T cells and therapies capable of re-inducing HLA class-I. Cancer Immunol Res 2014;2:27–36. 10.1158/2326-6066.CIR-13-0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gavvovidis I, Leisegang M, Willimsky G, et al. Targeting Merkel cell carcinoma by engineered T cells specific to T-antigens of Merkel cell polyomavirus. Clin Cancer Res 2018;24:3644–55. 10.1158/1078-0432.CCR-17-2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Longino NV, Yang J, Iyer JG, et al. Human CD4 + T cells specific for Merkel cell polyomavirus localize to Merkel cell carcinomas and target a required oncogenic domain. Cancer Immunol Res 2019;7:1727–39. 10.1158/2326-6066.CIR-19-0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sarma B, Willmes C, Angerer L, et al. Artesunate affects T antigen expression and survival of virus-positive Merkel cell carcinoma. Cancers 2020;12:12040919. 10.3390/cancers12040919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Samimi M, Molet L, Fleury M, et al. Prognostic value of antibodies to Merkel cell polyomavirus T antigens and VP1 protein in patients with Merkel cell carcinoma. Br J Dermatol 2016;174:813–22. 10.1111/bjd.14313 [DOI] [PubMed] [Google Scholar]
- 65. Lachance K, Akaike T, Cahill K, et al. 590 detecting Merkel cell carcinoma recurrence using a blood test: outcomes from 774 patients. J Invest Dermatol 2019;139:S101. 10.1016/j.jid.2019.03.666 [DOI] [Google Scholar]
- 66. Akaike G, Akaike T, Fadl SA, et al. Imaging of Merkel cell carcinoma: what imaging experts should know. Radiographics 2019;39:2069–84. 10.1148/rg.2019190102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Treglia G, Kakhki VRD, Giovanella L, et al. Diagnostic performance of fluorine-18-fluorodeoxyglucose positron emission tomography in patients with Merkel cell carcinoma: a systematic review and meta-analysis. Am J Clin Dermatol 2013;14:437–47. 10.1007/s40257-013-0040-x [DOI] [PubMed] [Google Scholar]
- 68. Singh N, Alexander NA, Lachance K, et al. Clinical benefit of baseline imaging in Merkel cell carcinoma: analysis of 584 patients. J Am Acad Dermatol 2021;84:330–9. 10.1016/j.jaad.2020.07.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. George A, Girault S, Testard A, et al. The impact of (18)F-FDG-PET/CT on Merkel cell carcinoma management: a retrospective study of 66 scans from a single institution. Nucl Med Commun 2014;35:282–90. 10.1097/MNM.0000000000000039 [DOI] [PubMed] [Google Scholar]
- 70. Moayed S, Maldjianb C, Adam R, et al. Magnetic resonance imaging appearance of metastatic Merkel cell carcinoma to the sacrum and epidural space. Magn Reson Imaging 2000;18:1039–42. 10.1016/s0730-725x(00)00176-4 [DOI] [PubMed] [Google Scholar]
- 71. Kong BY, Menzies AM, Saunders CAB, et al. Residual FDG-PET metabolic activity in metastatic melanoma patients with prolonged response to anti-PD-1 therapy. Pigment Cell Melanoma Res 2016;29:572–7. 10.1111/pcmr.12503 [DOI] [PubMed] [Google Scholar]
- 72. Sachpekidis C, Sidiropoulou P, Hassel JC. Positron emission tomography in Merkel cell carcinoma. Cancers 2020;12:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mahajan S, Barker CA, Mauguen A, et al. 18F-FDG PET/CT for post-treatment surveillance imaging of patients with stage III Merkel cell carcinoma. J Nucl Med 2021:jnumed.121.262882. 10.2967/jnumed.121.262882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Grotz TE, Joseph RW, Pockaj BA, et al. Negative sentinel lymph node biopsy in Merkel cell carcinoma is associated with a low risk of same-nodal-basin recurrences. Ann Surg Oncol 2015;22:4060–6. 10.1245/s10434-015-4421-7 [DOI] [PubMed] [Google Scholar]
- 75. Dubois M, Abi Rached H, Escande A, et al. Outcome of early stage Merkel carcinoma treated by exclusive radiation: a study of 53 patients. Radiat Oncol 2021;16:90. 10.1186/s13014-021-01815-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Waldinger R, Weinberg G, Gitman M. Local anesthetic toxicity in the geriatric population. Drugs Aging 2020;37:1-9. 10.1007/s40266-019-00718-0 [DOI] [PubMed] [Google Scholar]
- 77. Li G, Warner M, Lang BH, et al. Epidemiology of anesthesia-related mortality in the United States, 1999-2005. Anesthesiology 2009;110:759–65. 10.1097/aln.0b013e31819b5bdc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bhatia S, Storer BE, Iyer JG, et al. Adjuvant radiation therapy and chemotherapy in Merkel cell carcinoma: survival analyses of 6908 cases from the National cancer data base. J Natl Cancer Inst 2016;108:djw042. 10.1093/jnci/djw042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kaufman HL, Russell JS, Hamid O, et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer 2018;6. 10.1186/s40425-017-0310-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. D’Angelo SP, Lebbé C, Mortier L. First-line avelumab in a cohort of 116 patients with metastatic Merkel cell carcinoma (JAVELIN Merkel 200): primary and biomarker analyses of a phase II study. J Immunother Cancer 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Walker JW, Lebbé C, Grignani G, et al. Efficacy and safety of avelumab treatment in patients with metastatic Merkel cell carcinoma: experience from a global expanded access program. J Immunother Cancer 2020;8:e000313. 10.1136/jitc-2019-000313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ascierto PA, Orlova K, Grignani G, et al. Avelumab expanded access program in metastatic Merkel cell carcinoma: efficacy and safety findings from patients in Europe and the Middle East. Int J Cancer 2021;149:1926–34. 10.1002/ijc.33746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Grignani G, Chiarion Sileni V, Pinto C, et al. Avelumab treatment in Italian patients with metastatic Merkel cell carcinoma: experience from an expanded access program. J Transl Med 2021;19:70. 10.1186/s12967-021-02730-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nghiem P, Bhatia S, Brohl AS, et al. Two-year efficacy and safety update from JAVELIN Merkel 200 part A: a registrational study of avelumab in metastatic Merkel cell carcinoma progressed on chemotherapy. J Clin Oncol 2018;36:9507. 10.1200/JCO.2018.36.15_suppl.9507 [DOI] [Google Scholar]
- 85. Wrangle JM, Awad MM, Badin FB, et al. Preliminary data from QUILT 3.055: a phase 2 multi-cohort study of N803 (IL-15 superagonist) in combination with checkpoint inhibitors (CPI). J Clin Oncol 2021;39:2596. 10.1200/JCO.2021.39.15_suppl.2596 [DOI] [Google Scholar]
- 86. Rotondo JC, Bononi I, Puozzo A, et al. Merkel cell carcinomas arising in autoimmune disease affected patients treated with biologic drugs, including anti-TNF. Clin Cancer Res 2017;23:3929–34. 10.1158/1078-0432.CCR-16-2899 [DOI] [PubMed] [Google Scholar]
- 87. Bryant MK, Ward C, Gaber CE, et al. Decreased survival and increased recurrence in Merkel cell carcinoma significantly linked with immunosuppression. J Surg Oncol 2020;122:653–9. 10.1002/jso.26048 [DOI] [PubMed] [Google Scholar]
- 88. Kotteas EA, Pavlidis N. Neuroendocrine Merkel cell nodal carcinoma of unknown primary site: management and outcomes of a rare entity. Crit Rev Oncol Hematol 2015;94:116–21. 10.1016/j.critrevonc.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 89. Chen KT, Papavasiliou P, Edwards K, et al. A better prognosis for Merkel cell carcinoma of unknown primary origin. Am J Surg 2013;206:752–7. 10.1016/j.amjsurg.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 90. Vandeven N, Lewis CW, Makarov V, et al. Merkel cell carcinoma patients presenting without a primary lesion have elevated markers of immunity, higher tumor mutation burden, and improved survival. Clin Cancer Res 2018;24:963–71. 10.1158/1078-0432.CCR-17-1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lawrence LEB, Saleem A, Sahoo MK, et al. Is Merkel cell carcinoma of lymph node actually metastatic cutaneous Merkel cell carcinoma? Am J Clin Pathol 2020;154:369–80. 10.1093/ajcp/aqaa051 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-004742supp001.pdf (9.9MB, pdf)