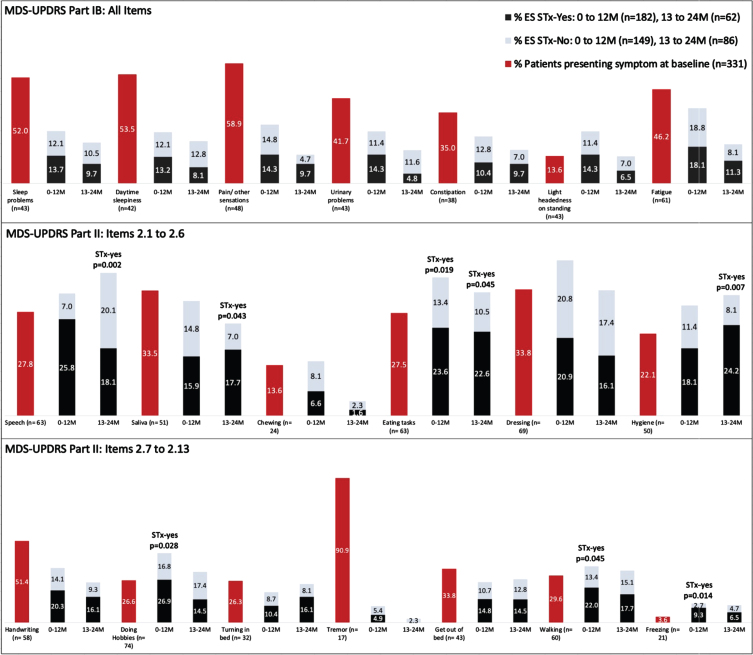
Fig. 1.
Percentages of participants endorsing individual MDS-UPDRS Part IB and II scale items at baseline and at follow-up study visits. Emergent symptoms (ES) reported at the follow-up timepoints are divided according to use of antiparkinson therapy (STx-yes and STx-no). Once a participant endorsed a given symptom, they were no loger included in the denominator for that scale item. Thus, the numbers reflect the proportions of participants available at the respective time points who had not previously reported the symptom, so the totals for each item do not sum to 100%.