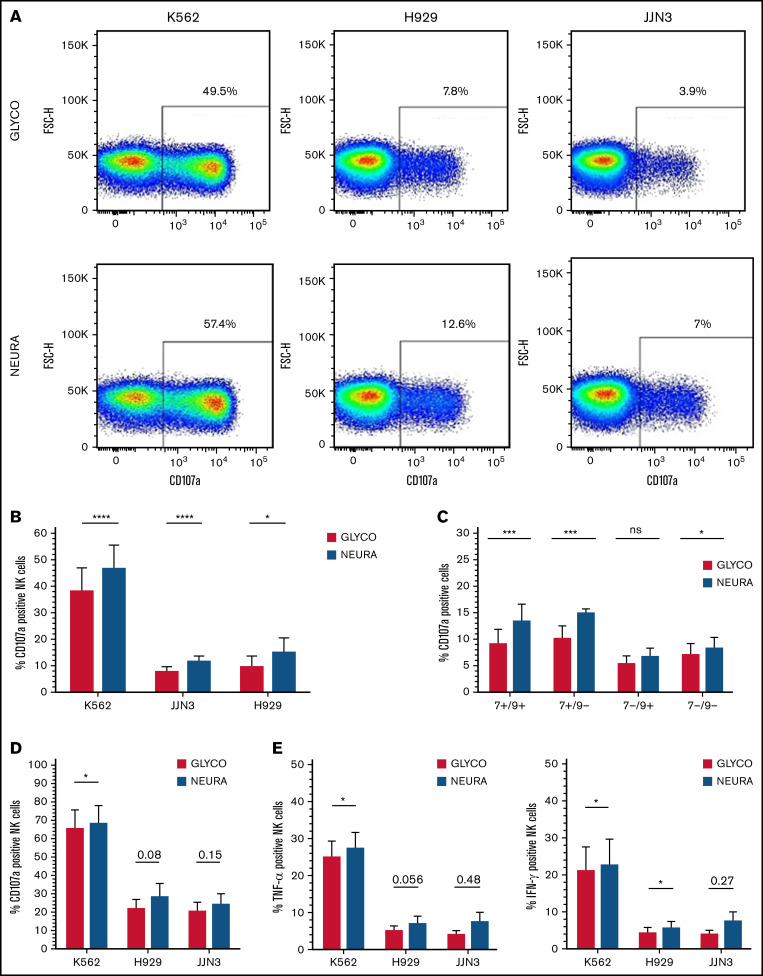
Figure 4.
Desialylation of MM cells increases NK cell degranulation and surface-expressed CD107a after coculture. (A) IL-2 activated NK cells were cocultured with K562, JJN3, or H929 ± desialylation using NEURA or GLYCO for 1 hour, after which cells were collected and CD107a expression was measured on bulk NK cells. Histogram representative of n = 1 biological repeat for each cell under each condition. (B) CD107a expression on IL-2 activated primary NK cells exposed to NEURA-treated K562, JJN3, and H929 was determined and compared with CD107a expression on NK cells cocultured with GLYCO-treated controls. (C) NK cells were subdivided based on Siglec-7/Siglec-9 expression and subset degranulation were measured after coculture with JJN3 treated with either GLYCO or NEURA. (D) CD107a expression was measured on expanded primary NK cells exposed to NEURA-treated K562, JJN3, and H929 and compared with CD107a expression on NK cells cocultured with GLYCO-treated controls. (E) TNF-α and IFN-γ expression within NK cells was measured after coculture with NEURA-treated K562, JJN3, and H929 and compared with the expression of TNF-α and IFN-γ when cocultured with GLYCO-treated controls. (B-E) Data analyzed using Student’s paired t-test; graphs represent mean CD107α/TNF-α/IFN-γ positive NK cells +SEM. An individual repeat of n = 7 donors (A), n = 7 (B-C), n = 5 (D-E). *P < .01; ***P < .001; ****P < .0001; ns, not significant.