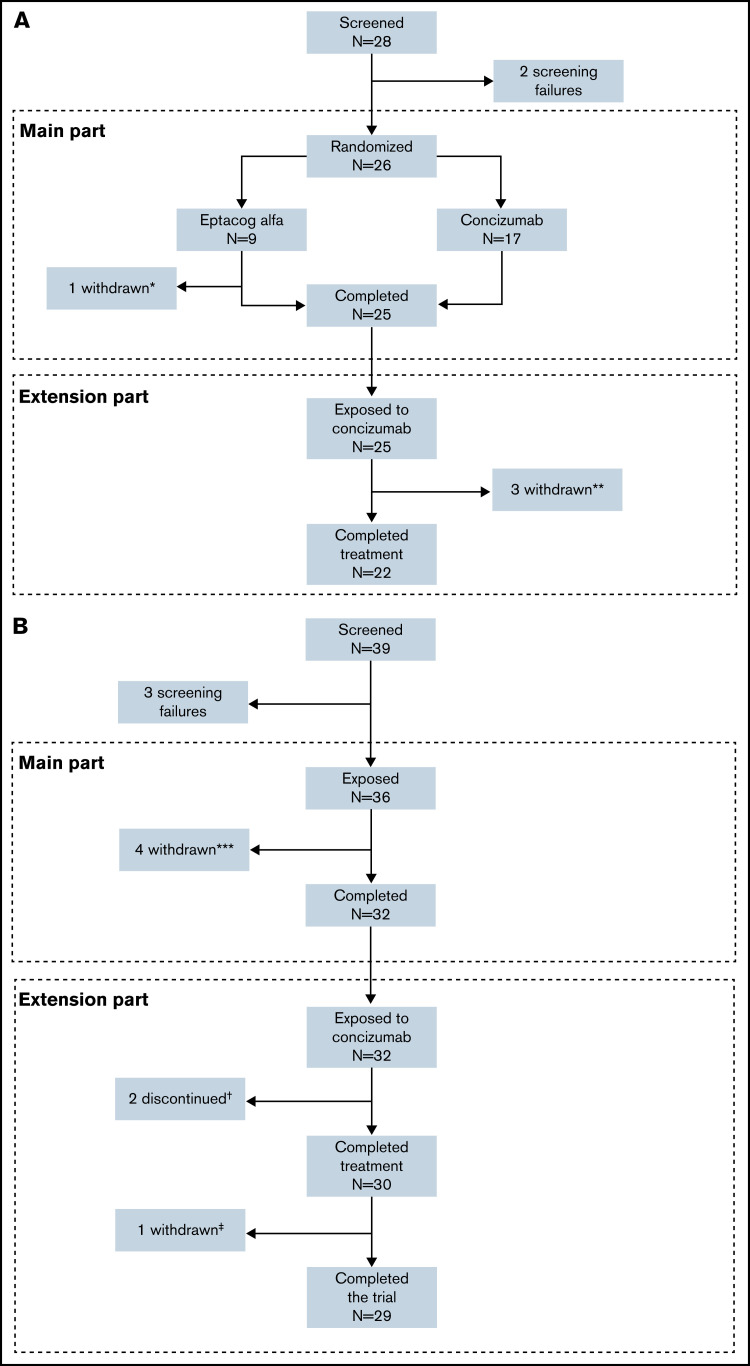
Figure 2.
Patient disposition in the phase 2 concizumab trials. (A) explorer4 (HAwI, HBwI). (B) explorer5 (HA). *One patient withdrew consent after randomization. **Three patients withdrew in the extension phase (one due to lack of efficacy, one because of suspicion of no therapeutic effect due to normal TFPI level with ADA, and one withdrawal of consent). ***Four patients withdrew before the end of the main part. †Two patients discontinued due to lack of efficacy. ‡One patient withdrew consent in the extension part.