Figure 3: Sample resulting flowchart.
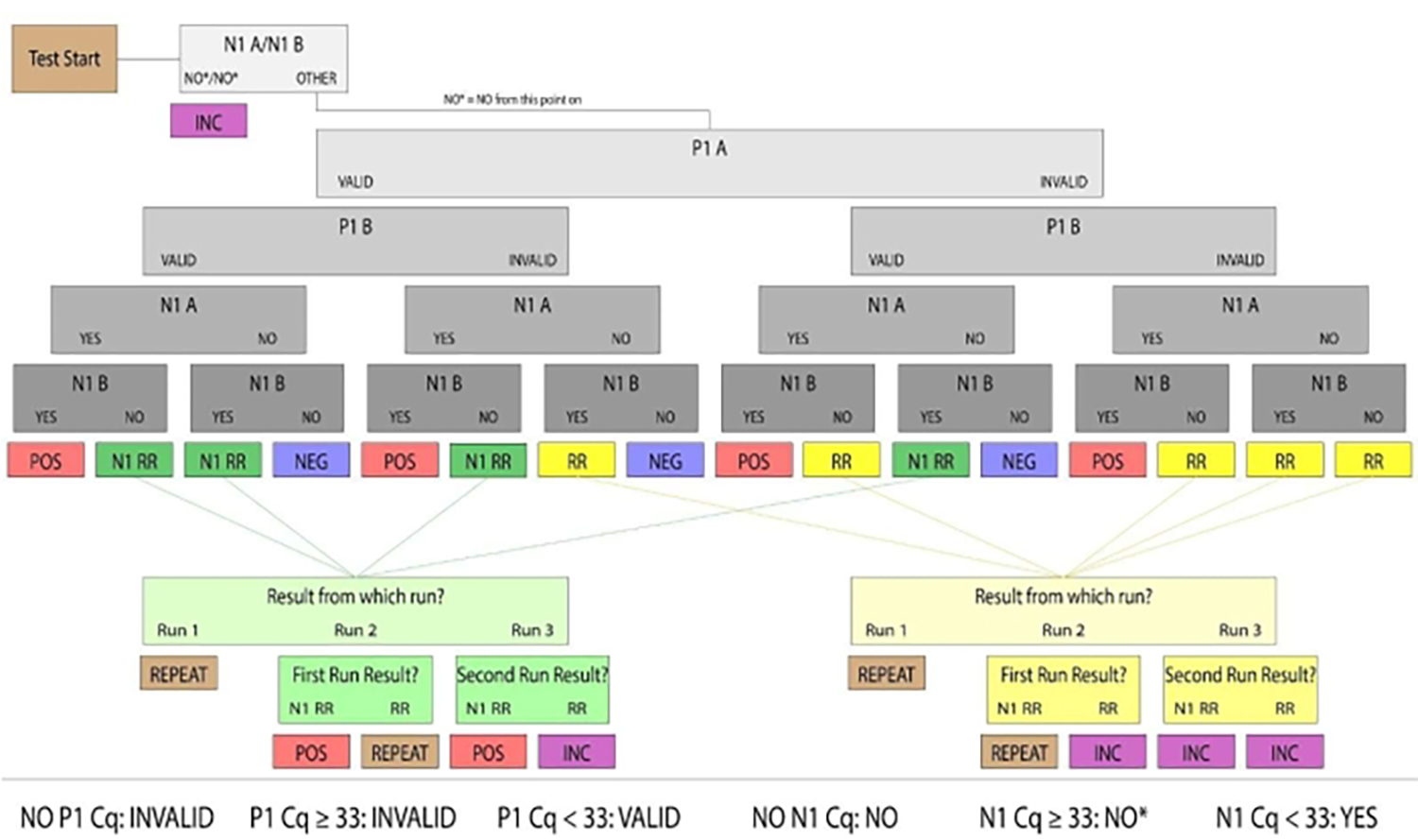
Samples with valid P1 and positive N1 were determined to be human saliva samples positive for SARS-CoV-2. Valid and positive/negative sample results were considered conclusive. Samples that did not produce conclusive results in the first run were categorized as Rerun (denoted RR) or N1 Rerun (denoted N1 RR). Rerun samples had no valid P1 amplification, and N1 Rerun samples had positive N1 amplification in a single replicate. If no valid P1 amplification could be produced by a subsequent manual run, or both replicates had N1 Ct values above the positive threshold (Ct >33), the sample results were considered inconclusive. For clinical purposes, patient samples that did not arrive at the lab, had an insufficient quantity of saliva to pipette or were damaged were considered invalid.
