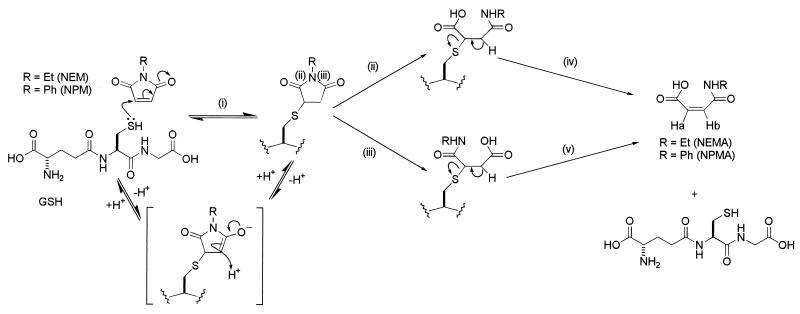
FIG. 5.
Possible mechanism for the detoxification of N-substituted maleimides in E. coli. (i) Spontaneous nucleophilic Michael attack of the cysteine lone pair in GSH on the NEM to give the hemithioacetal ESG. This reaction is reversible. (ii and iii) Hydrolysis of the ESG (at the positions marked) by a putative imidase. The reaction can occur at both sites to give the intermediates shown, both giving the same final product. (iv and v) Elimination of GSH from both intermediates to form the double bond in NEMA and NPMA. The NMR chemical shifts for Ha and Hb are given in Table 1.