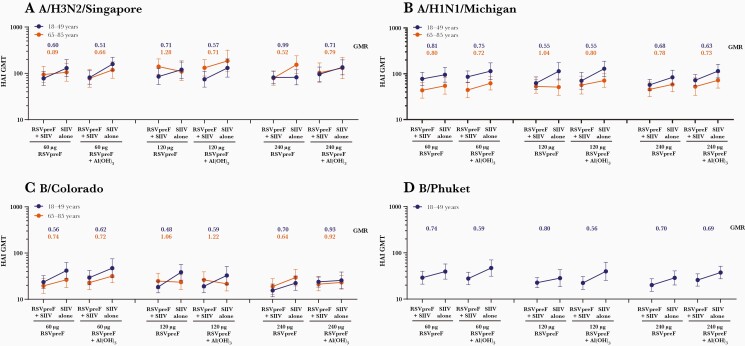
Figure 5.
Hemagglutination inhibition (HAI) geometric mean titers to A/H3N2/Singapore (A), A/H1N1/Michigan (B), B/Colorado (C), and B/Phuket (D) at 1 month after seasonal inactivated influenza vaccine (SIIV) in participants aged 18–49 and 65–85 years receiving either SIIV concomitantly with bivalent respiratory syncytial virus prefusion F vaccine (RSVpreF) (RSVpreF + SIIV) or SIIV 1 month after RSVpreF (SIIV alone) in the expanded cohort (evaluable influenza immunogenicity population). Total number of participants = 31–41 per group. Geometric mean ratios at 1 month after SIIV for concomitant (RSVpreF + SIIV) to sequential (SIIV alone) administration are shown above each bar. Participants 18–49 years of age received quadrivalent SIIV containing A/H3N2/Singapore, A/H1N1/Michigan, B/Colorado, and B/Phuket; participants 65–85 years of age received high-dose trivalent SIIV containing A/H3N2/Singapore, A/H1N1/Michigan, and B/Colorado. Abbreviations: Al(OH)3, aluminum hydroxide; GMR, geometric mean ratio; GMT, geometric mean titer; HAI, hemagglutination inhibition; RSVpreF, bivalent respiratory syncytial virus prefusion F vaccine; SIIV, seasonal inactivated influenza vaccine.