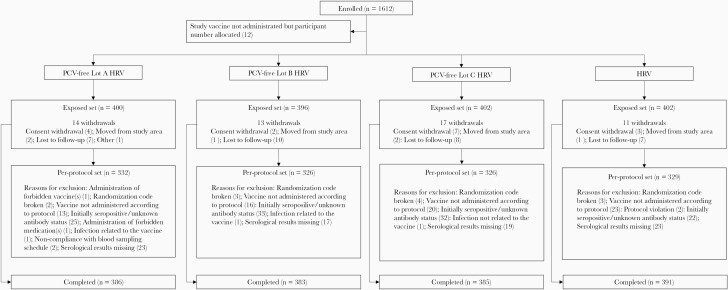
Figure 2.
Flow of participants. Abbreviations: HRV, group receiving human rotavirus vaccine licensed at the time of study conduct; n, number of infants in each group; PCV-free Lot A HRV, PCV-free Lot B HRV, PCV-free Lot C HRV, groups receiving the porcine circovirus-free human rotavirus vaccine from lots A, B, or C.