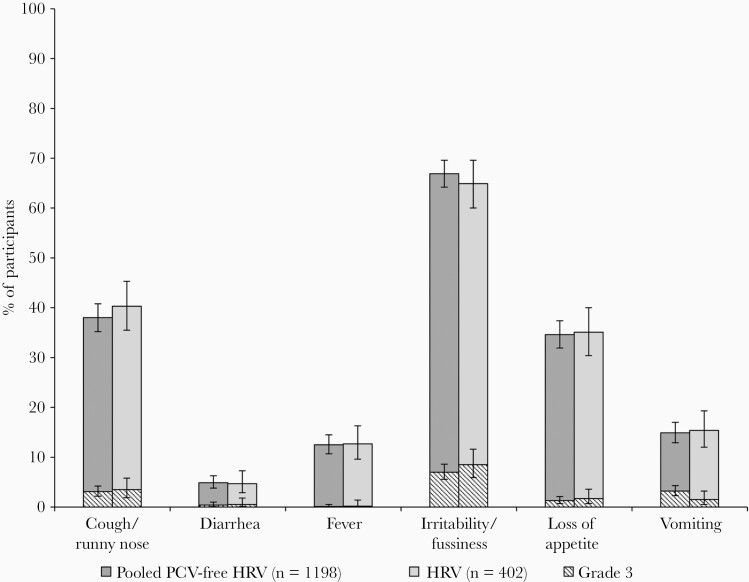
Figure 3.
Solicited general adverse events after HRV vaccination, overall per infant (exposed set). Grade 3 was defined as preventing normal daily activity (irritability/fussiness and cough/runny nose), did not eat at all (loss of appetite), ≥6 looser than normal stools/day (diarrhea), ≥3 episodes of vomiting/day (vomiting), and body temperature >39.5°C (fever). Abbreviations: HRV, human rotavirus vaccine licensed at the time of study conduct; Pooled PCV-free HRV, pooled groups receiving the porcine circovirus-free human rotavirus vaccine.