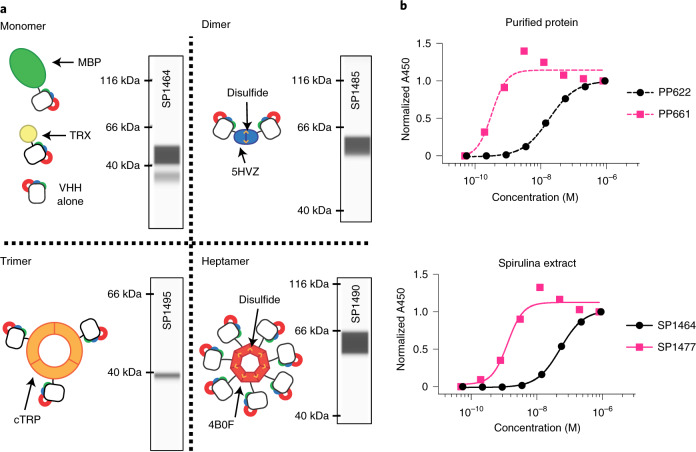
Fig. 2. VHH scaffolding strategies.
a, Cartoons of multimeric scaffolds with sample expression data for VHHs in spirulina. Monomeric (MBP and thioredoxin (TRX)), dimeric (5HVZ), trimeric (cTRP) and heptameric (4B0F) scaffolding proteins were used to multimerize VHHs expressed in spirulina. Intersubunit disulfides confer additional stability to the dimeric and heptameric scaffolds, and these forms were commonly expressed with an MBP tag to improve solubilization. Inset CEIA blots for each scaffold demonstrate spirulina expression of a SARS-CoV-2 RBD-binding VHH49 fused to the indicated scaffolding protein. CEIA analysis was performed performed once for each strain. All proteins were observed at the appropriate size. b, Increase in apparent binding activity by dimerization of VHHs, as measured by ELISA with purified VHH (top) and spirulina extract (bottom). E. coli-expressed and purified monomeric (PP622) and dimeric (PP661) forms of RBD-binding VHH were assayed with RBD and compared with the binding activity of identical proteins present in spirulina extracts (SP1464 and SP1477, respectively). The concentration of VHH in the spirulina extracts was determined by CEIA. ELISA was performed once with duplicate samples. Absorbance was normalized to the highest absorbance within each sample. EC50 values of 18.3 and 52.3 nM were recorded for monomeric VHH in purified (PP622) and extract (SP1464) samples, respectively, while those for dimeric VHH were 0.32 and 1.29 nM for the purified (PP661) and extracted forms (SP1477), respectively.