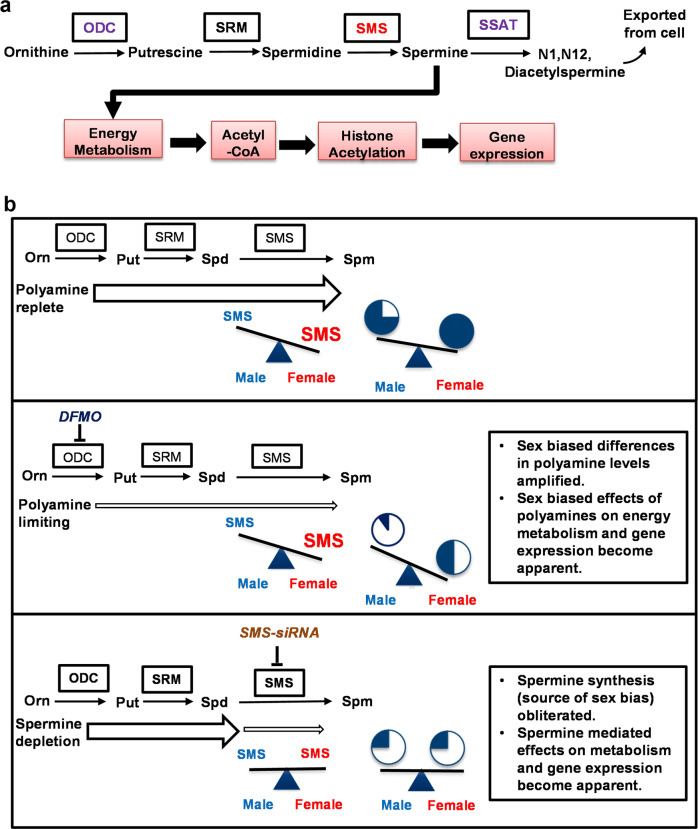
Fig. 7. Schematic overview of polyamine metabolism and the mechanism of polyamine-mediated gene expression.
a Polyamine metabolic pathway with key enzymes highlighted and the downstream effects on trophoblast function. Spermine regulates cellular energy metabolism to increase acetyl-coA availability for histone acetylation and gene expression. Inhibition of ODC with DFMO, silencing of SMS via siRNAs, or activating SSAT using DENSPM decreases spermine levels leading to dysregulation of energy metabolism, histone acetylation, and gene expression. b Model of the effects of sex-biased polyamine metabolism on trophoblast function. Top panel: under physiological conditions, the female-biased SMS expression results in modest changes to spermine synthesis which are unlikely to result in sex-biased effects of polyamines on trophoblast function. Middle panel: Under polyamine limiting conditions the female bias in spermine synthesis buffers the effects of polyamine depletion leading to marked sex differences in spermine levels and sex-specific regulation of trophoblast function. Bottom panel: Upon spermine depletion, the effects on metabolism and gene expression become apparent but the sex-dependent effects are not observed because the source of the sex-bias is no longer present. Spermine levels are represented by the pie-charts.