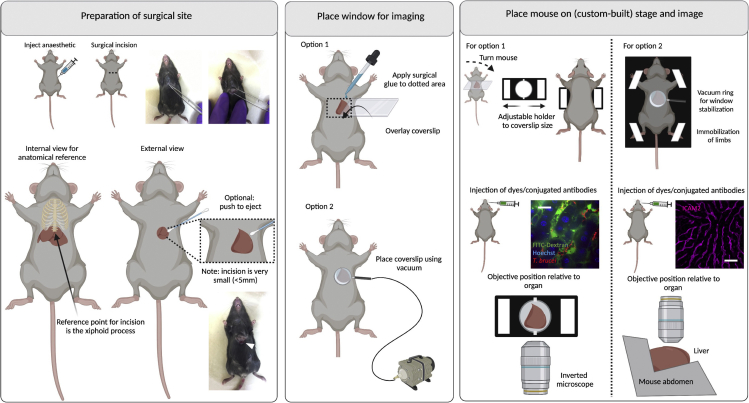
Figure 8.
Surgeries for implanting terminal windows in the mouse liver
Left panel: Preparation of surgical site. Begin by injecting anesthetic intraperitoneally. Diagram and picture show site of incision. Find the xiphoid process (at the bottom of the ribs) to locate the site for incision. Make a small incision (up to 5 mm) and with the aid of cotton buds, eject either the middle or part of the left liver lobe. Middle panel: Place window for imaging. Two options exist for imaging the liver either using an inverted microscope (option 1) or an upright microscope (option 2). Option 1: Use a rectangular coverslip and secure on the mouse using surgical glue. Option 2: Use a round coverslip attached to a vacuum ring and place on top of the incision. Right panel: Place mouse on stage and image. Option 1: For imaging in an inverted microscope, an adjustable holder can be used on the microscope stage. The mouse is turned to display the ventral side (and the incision), and the coverslip attached to the mouse is secured on the holder. Note: This setup will likely be sufficient for a mouse of 6–10 weeks old. Younger mice will likely need support for the head and lower body. Older mice will likely bend the coverslip, risking that it breaks within the microscope. For older mice, option 2 is therefore recommended. The objective reaches the liver from the bottom.Option 2: A custom-built stage provides full body support to the mouse and allows space for tubing (used for vacuum, and intravenous cannulation). Immobilize all limbs and tail. Depending on the experimental conditions, dyes can be added intravenously either before or after securing the mouse on the stage. The objective reaches the liver from the top. Scale bar: 20 μm. Created with BioRender.com.