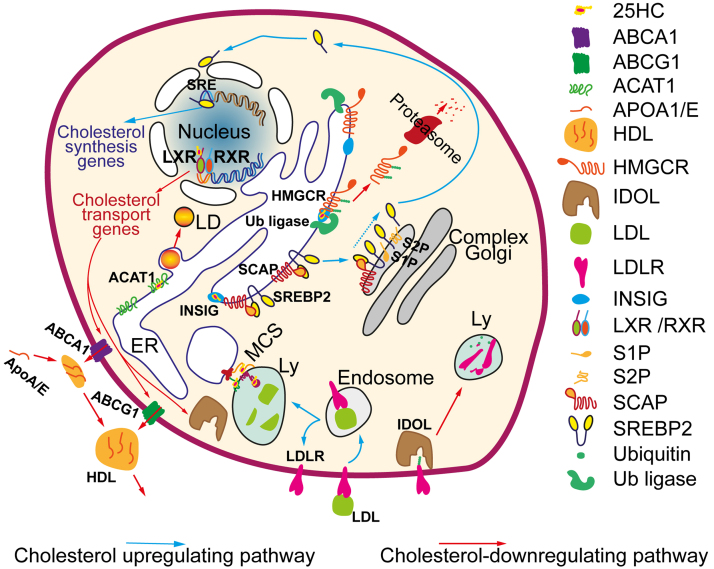
Fig. 2.
25-HC-dependent regulation of cellular cholesterol homeostasis. Excessive cholesterol is converted to 25-HC in the ER, where 25-HC binds to INSIG. (i) INSIG-25-HC promotes the retention of the SCAP-SREBP2 complex in the ER. In the absence of 25-HC, the SCAP-SREBP2 complex relocates to the Golgi apparatus, where SREBP2 undergoes proteolytic cleavage by the S1P and S2P proteases, releasing the active transcription factor SREBP2. SREBP2 binds to the regulatory regions (sterol regulatory elements, or SREs) of cholesterol biosynthesis genes and activates their expression [45, 46]. (ii) In the presence of 25-HC, INSIG binds to ubiquitin ligases, which promote ubiquitination of HMGCR, the rate-limiting enzyme of cholesterol synthesis. The ubiquitinated enzyme is then directed to proteasomal degradation [44]. 25-HC stimulates LXRs [3, 42], which activate expression of genes of ABC transporters (ABCA1, ABCG1) and ubiquitin ligase IDOL [49, 50]. (iii) ABC transporters efflux cellular cholesterol to the APO-A/E proteins, thus mediating formation and maturation of lipoprotein particles [42]. (iv) IDOL ubiquitinates LDL receptors and promotes their translocation to the lysosomes for proteolysis; as a result, the LDL receptor-mediated uptake of extracellular cholesterol decreases [49]. (v) 25-HC interacts with the proteins that organize membrane contact sites (MCSs), thus reducing the transport of cholesterol between organelles [41, 51, 52]. (vi) 25-HC directly activates ACAT1-dependent esterification of cholesterol in the ER, facilitating the deposition of excessive cholesterol in the lipid droplets (LDs) [47, 53]. Blue and red arrows show positive and negative regulation of cholesterol levels, respectively.