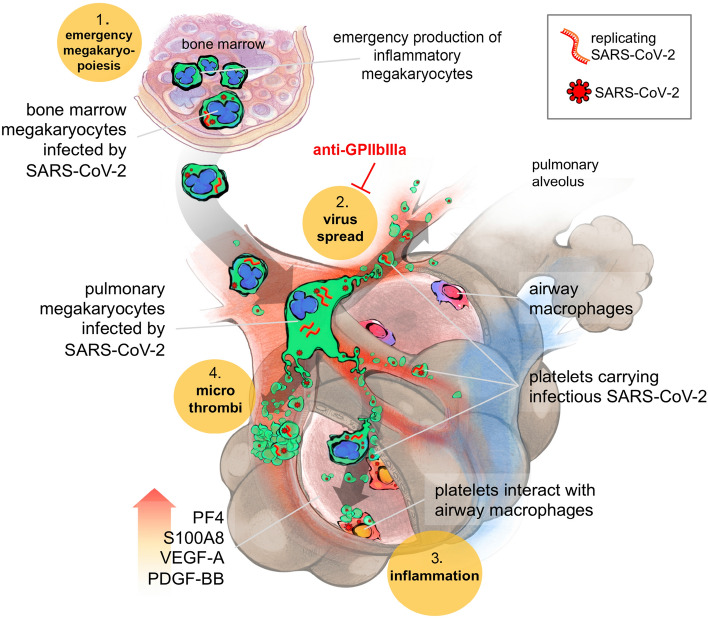
Fig. 5.
Scheme: Platelets harboring SARS-CoV-2 offer a convergent therapeutical target in severe COVID-19 with multiple manifestations. 1- SARS-CoV-2 favors emergency inflammatory megakaryopoiesis. SARS-CoV-2 infected MKs in the bone marrow (containing both viruses and replicating SARS-CoV-2 (-) RNA) migrate to the lungs where they contribute to thrombopoiesis and produce SARS-CoV-2-containing platelets in the pulmonary circulation. 2- These infectious platelets will then spread the virus and contribute to the systemic inflammatory component of severe COVID-19. As platelets sheltering SARS-CoV-2 are coated with von Willebrand Factor, indicating their highly activated status, they will also contribute to thrombus formation typical of COVID-19 complications. 3- Increase in lung VEGF-A and PDGF-BB participates to alveolar endothelial destruction and effraction allowing platelets carrying SARS-CoV-2 to reach and infect alveolar macrophages. 4- Increased lung PF4/CXCL4 released by platelets and S100A8 likely contribute to the maintenance of a highly inflammatory environment, macrophage activation, and cytokine storm. These four platelet-mediated components of severe COVID-19 suggest that targeting platelets, with the use of anti-platelet drugs like anti-GPIIbIIIa, might be an efficient strategy to block viral spread, thrombus formation and exacerbated inflammation at once, increasing the chance of survival