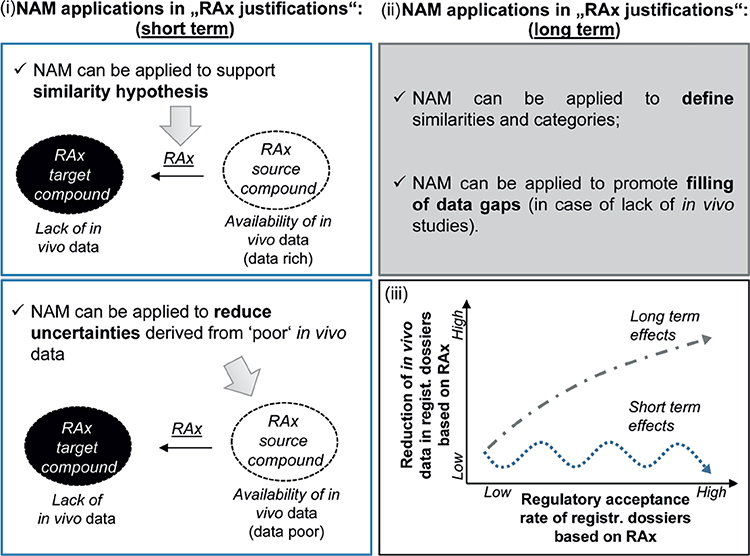
Fig. 4: Inclusion of NAM-derived data in RAx justification dossiers to build confidence in its regulatory use.
(i) A registration dossier based on RAx always contains a justification including data that prove the validity of the similarity hypothesis and/or the reliability of the in vivo studies performed on the source compound. Recently, NAM-derived data have started to be used as complimentary information to support the RAx hypothesis. (ii) An increasing confidence in the use of NAM-data may lead to their exclusive use for similarity definition, category formation, and to fill data gaps (in case of lack of in vivo data). (iii) As short-term effect, the use of NAM-derived data in RAx dossiers can increase the regulatory acceptance rate of RAx justifications in risk assessment. As long-term effect, the increasing confidence in such data could lead to a RAx application based only on in vitro/in silico data.